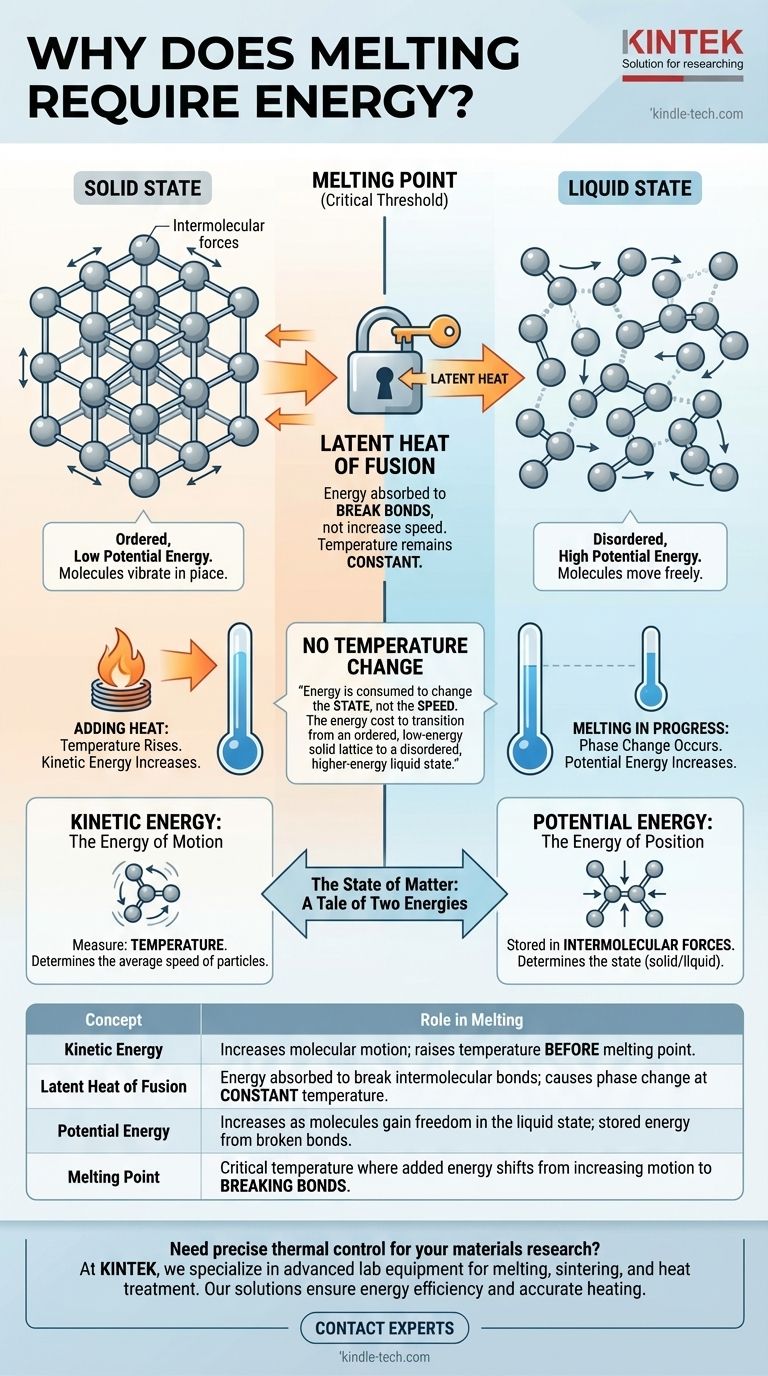
At the molecular level, melting requires energy because that energy is used to break the rigid bonds holding particles together in a fixed solid structure. This energy, known as latent heat, does not increase the speed of the molecules—and therefore doesn't raise the temperature—but instead increases their potential energy, giving them the freedom to move and slide past one another as a liquid.
The core reason melting requires energy without a temperature change is that the energy is consumed to change the state, not the speed, of the molecules. It's the energy cost to transition from an ordered, low-energy solid lattice to a disordered, higher-energy liquid state.

The State of Matter: A Tale of Two Energies
To understand the energy of melting, we must first distinguish between the two types of energy molecules possess: kinetic and potential.
Kinetic Energy: The Energy of Motion
Kinetic energy is the energy of movement. In a substance, this manifests as the vibration, rotation, and translation of its constituent atoms or molecules.
The temperature of a substance is a direct measure of the average kinetic energy of its particles. When you add heat and the substance gets hotter, it's because its molecules are moving faster.
Potential Energy: The Energy of Position
Potential energy, in this context, is the energy stored in the forces between molecules, known as intermolecular forces. It is determined by the arrangement and separation of these particles.
A tightly bound, ordered structure like a solid has low potential energy. A disordered, free-flowing structure like a liquid has higher potential energy because the molecules are further apart and less constrained.
The Solid State: A Highly Ordered Structure
In a solid, particles are locked into a fixed, repeating pattern called a crystal lattice. They are held in place by strong intermolecular forces.
While they aren't motionless, their kinetic energy is limited to vibrating in place. They lack the energy to overcome the forces holding them in this rigid structure.
Demystifying the Melting Process
The phase change from solid to liquid happens in a distinct, stepwise process where energy is allocated to a very specific task.
Adding Heat: The First Stage
When you begin to add heat to a solid (like ice below its freezing point), the energy is absorbed as kinetic energy. The particles vibrate more and more intensely within the lattice.
During this stage, the temperature of the solid rises steadily.
Reaching the Melting Point: A Critical Threshold
The melting point is the temperature at which the particles are vibrating so vigorously that they are on the verge of breaking free from the lattice.
At this exact temperature, a fundamental shift occurs. Any additional energy added no longer increases the vibration speed (kinetic energy). Instead, it is directed entirely toward overcoming the intermolecular forces.
The Role of Latent Heat of Fusion
The energy required to change a substance from a solid to a liquid at its melting point is called the latent heat of fusion. The word "latent" means hidden, because this energy input does not result in a temperature change.
Think of it as using a key to unlock a cage. The energy isn't making the inhabitant run faster inside the cage; it's being used exclusively to open the lock and allow it to get out.
From Order to Disorder: The Liquid State
As molecules absorb enough latent heat, they break free from their fixed positions in the lattice. The rigid structure collapses, and the substance becomes a liquid.
In this new liquid state, the particles have much higher potential energy, but their average kinetic energy (temperature) is the same as the solid it just melted from. Only after all the solid has melted will further heat addition begin to increase the kinetic energy and thus the temperature of the liquid.
Understanding the Key Distinction: Heat vs. Temperature
The concept of latent heat resolves a common point of confusion. It highlights the difference between adding energy and observing a temperature increase.
The Common Misconception
Many people wonder, "If I'm still adding heat to melting ice, why does the temperature stay at 0°C (32°F)?"
The answer is that the energy has a different job to do during a phase change. Its priority is to break bonds, not to increase speed.
Heat is Energy Transfer, Temperature is a Measurement
Heat is the transfer of thermal energy. Temperature is a measurement of the average kinetic energy.
During melting, you are continuously transferring heat into the system, but that energy is being converted into potential energy, leaving the average kinetic energy—and thus the temperature—unchanged.
How to Apply This Principle
Understanding this concept is fundamental to many real-world applications and scientific fields.
- If your primary focus is everyday observation (like an ice cube in a drink): The energy being absorbed from the warmer liquid is used as latent heat to break the ice's molecular bonds, which is why the drink gets colder while the ice itself melts at a constant temperature.
- If your primary focus is engineering or materials science: The specific latent heat of fusion is a critical property that determines the energy cost to melt metals or other materials, directly impacting furnace design, energy consumption, and process efficiency.
- If your primary focus is chemistry or physics: Remember that phase changes represent a shift in potential energy (due to changes in intermolecular forces), while temperature changes represent a shift in kinetic energy (due to changes in molecular motion).
Grasping the role of latent heat is the key to understanding how energy drives changes in the physical state of all matter.
Summary Table:
| Concept | Role in Melting |
|---|---|
| Kinetic Energy | Increases molecular motion; raises temperature before melting point. |
| Latent Heat of Fusion | Energy absorbed to break intermolecular bonds; causes phase change at constant temperature. |
| Potential Energy | Increases as molecules gain freedom in the liquid state; stored energy from broken bonds. |
| Melting Point | Critical temperature where added energy shifts from increasing motion to breaking bonds. |
Need precise thermal control for your materials research? At KINTEK, we specialize in advanced lab equipment that delivers accurate heating and temperature management for processes like melting, sintering, and heat treatment. Whether you're working with metals, ceramics, or other materials, our solutions ensure energy efficiency and reproducible results. Contact our experts today to find the perfect furnace or heating system for your laboratory's needs.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the process variables of sintering? Master Temperature, Time, Pressure & Atmosphere
- What is the current of sputtering ion? Control Your Thin Film Deposition Rate and Quality
- Why is the use of a high-temperature drying oven necessary for aluminum sludge recycling? Ensure Data Precision
- What metals can be sputter coated? Unlock the Potential of Virtually Any Metal or Alloy
- What are the raw materials for bio-oil? A Guide to Selecting the Best Biomass Feedstock
- What is the role of a laboratory drying oven in catalyst treatment? Ensure Structural Integrity & High Performance
- What is the temperature of a plasma furnace? Unlocking Extreme Heat for Demanding Applications
- How do you ensure the safe operation of equipment and machinery? A Proactive Guide to Risk Management



















