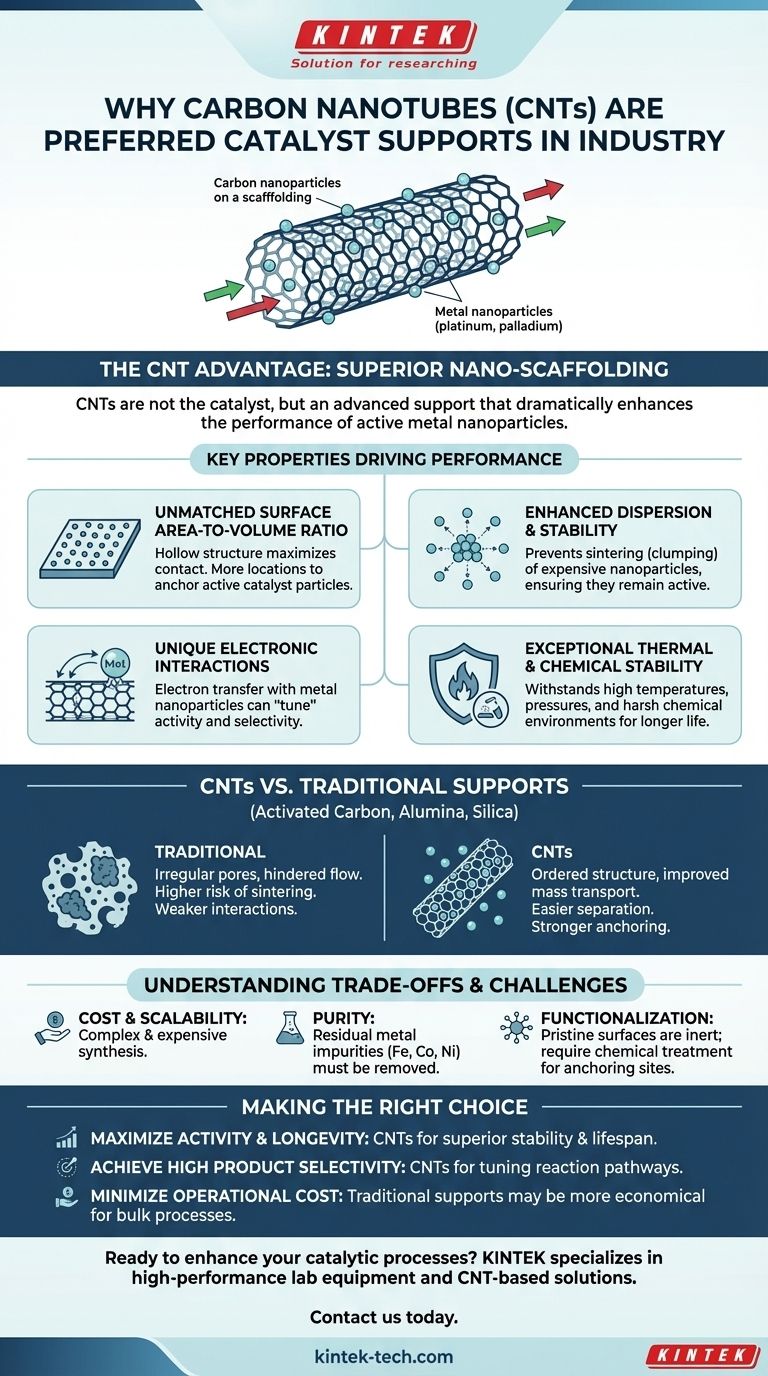
The preference for carbon nanotubes (CNTs) in catalysis stems from their exceptional physical structure and unique electronic properties. They are not typically the catalyst themselves, but rather a superior support material that dramatically enhances the performance of active catalyst particles, like metal nanoparticles. This nano-scaffolding provides a massive surface area and prevents the catalyst from deactivating under harsh industrial conditions.
The true value of carbon nanotubes in catalysis is their function as an advanced support structure. They immobilize expensive catalyst particles, prevent them from clumping together, and can even electronically influence the reaction to improve efficiency and selectivity far beyond what traditional supports can offer.

The Unique Properties Driving CNT Catalysis
To understand why CNTs are so effective, we need to look beyond their simple chemical composition and examine their nanoscale architecture and quantum-level electronic behavior. These factors combine to create a near-ideal environment for catalytic activity.
Unmatched Surface Area-to-Volume Ratio
A core principle of catalysis is maximizing the contact area between the catalyst and the reactants. The hollow, cylindrical structure of CNTs provides an enormous specific surface area.
This means that for a given mass, CNTs offer significantly more locations to anchor active catalyst particles compared to conventional flat or porous materials.
Enhanced Catalyst Dispersion and Stability
One of the most common failure modes for industrial catalysts is sintering, where expensive metal nanoparticles (like platinum or palladium) migrate and clump together at high temperatures. This clumping reduces the available surface area and deactivates the catalyst.
CNTs excel at preventing this. Their surface can be functionalized with defects or chemical groups that act as powerful anchoring sites. These sites firmly hold the metal nanoparticles in place, ensuring they remain small, separated, and highly active throughout the reaction cycle.
Unique Electronic Interactions
Unlike inert supports like silica or alumina, CNTs are electronically active. Their structure, made of sp²-hybridized carbon atoms, creates a cloud of delocalized pi-electrons.
This allows the CNT support to engage in electron transfer with the metal nanoparticles it holds. This interaction can modify the electronic state of the metal, effectively "tuning" its catalytic activity and selectivity for a specific desired chemical transformation.
Exceptional Thermal and Chemical Stability
The strong carbon-carbon covalent bonds in the nanotube lattice make them incredibly robust.
CNTs can withstand the high temperatures, high pressures, and corrosive chemical environments (both acidic and basic) common in industrial reactors. This durability translates directly to a longer catalyst lifetime and less frequent replacement.
CNTs vs. Traditional Catalyst Supports
The advantages of CNTs become clear when compared directly to the materials they are designed to replace.
The Problem with Conventional Supports
Traditional supports like activated carbon, alumina, and silica have served the industry for decades but come with inherent limitations.
Activated carbon has a high surface area but an irregular pore structure that can hinder the flow of reactants. Alumina and silica are often more inert and have weaker interactions with metal particles, leading to a higher risk of sintering.
The CNT Advantage in Practice
CNTs offer a more ordered structure, which improves mass transport, allowing reactants to easily reach the active sites and products to easily leave.
Furthermore, their unique fibrous shape can make them easier to separate from a liquid reaction mixture, simplifying product purification and catalyst recycling.
Understanding the Trade-offs and Challenges
Despite their clear technical advantages, CNTs are not a universal solution. Their adoption is limited by several practical and economic factors that must be considered.
The Issue of Cost and Scalability
The synthesis of high-purity, structurally consistent CNTs remains significantly more expensive and complex than producing bulk materials like activated carbon or silica. This initial cost can be a major barrier for large-scale industrial applications.
Purity and Residual Metals
The most common methods for producing CNTs rely on metal catalysts themselves, typically iron, cobalt, or nickel. Traces of these residual metals can remain in the final CNT product.
If not thoroughly removed through purification, these impurities can interfere with or poison the intended catalytic reaction, leading to unwanted byproducts.
The Need for Functionalization
In their pristine, as-produced state, CNT surfaces are relatively smooth and chemically inert. This makes it difficult for catalyst nanoparticles to adhere strongly.
To create the necessary anchoring sites, CNTs must often undergo a process called functionalization. This typically involves harsh acid treatments to create defects and attach oxygen-containing groups, adding another step of complexity and cost to the process.
Making the Right Choice for Your Application
Deciding whether to use a CNT-based catalyst requires a careful analysis of performance requirements versus practical and economic constraints. The right choice depends entirely on your primary goal.
- If your primary focus is maximizing catalytic activity and longevity: CNTs are a superior choice, as their ability to prevent nanoparticle sintering and provide a massive surface area leads to a longer and more active catalyst lifetime.
- If your primary focus is achieving high product selectivity: The unique electronic properties of CNTs can be leveraged to tune the reaction pathway, making them ideal for complex syntheses where specific outcomes are critical.
- If your primary focus is minimizing operational cost for a bulk chemical process: Traditional supports may still be more economical, unless the performance gains from CNTs provide a return that justifies the higher initial investment.
Ultimately, carbon nanotubes represent a powerful platform for designing next-generation catalysts, offering an unprecedented level of control over reactivity, selectivity, and stability.
Summary Table:
| Feature | CNT Advantage | Benefit |
|---|---|---|
| Surface Area | High specific surface area | Maximizes active sites for catalyst particles |
| Stability | Resists sintering and harsh conditions | Longer catalyst lifespan, reduced replacement |
| Electronic Properties | Enables electron transfer with catalysts | Improves reaction selectivity and efficiency |
| Structure | Ordered, fibrous morphology | Enhanced mass transport and easier separation |
Ready to enhance your catalytic processes with advanced carbon nanotube solutions? KINTEK specializes in high-performance lab equipment and consumables, including CNT-based catalysts tailored for laboratory and industrial needs. Our products are designed to deliver superior stability, selectivity, and efficiency for your chemical reactions. Contact us today to discuss how our solutions can optimize your workflow and drive innovation in your research or production!
Visual Guide
Related Products
- CVD Diamond Domes for Industrial and Scientific Applications
- Graphite Vacuum Continuous Graphitization Furnace
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
People Also Ask
- What is chemical Vapour deposition in simple words? A Simple Guide to 'Painting' with Gas
- What is a thin film technology? The Atomic-Scale Process Powering Modern Electronics
- Why is a high-precision rotating sample holder necessary for Al-Zr coatings? Ensure Uniformity and Precision
- What is the precursor for CNT preparation? Choosing the Right Carbon Source for Quality & Yield
- What are the parameters for chemical vapour deposition? Master Temperature, Pressure & Gas Flow for Perfect Films
- What are the ingredients in synthetic diamonds? Unlocking the Science of Lab-Grown Carbon Crystals
- What is modified chemical vapour deposition method? The Inside-Out Process for Ultra-Pure Optical Fibers
- What is the process of thermal CVD? A Step-by-Step Guide to High-Purity Thin Film Deposition



















