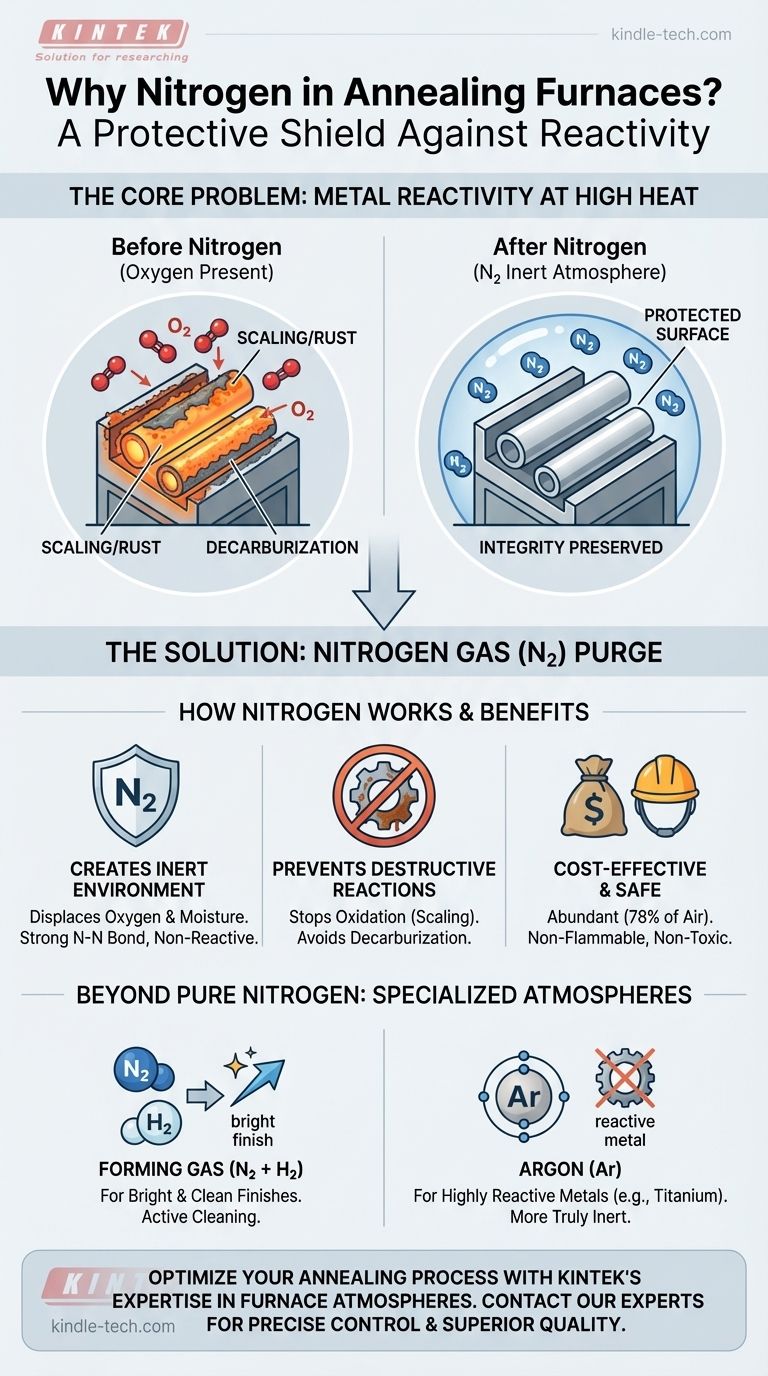
In short, nitrogen is used in an annealing furnace to create a protective, non-reactive atmosphere. This inert environment displaces oxygen and moisture, preventing destructive chemical reactions like oxidation (scaling/rust) and decarburization from occurring on the metal's surface at high temperatures.
The core challenge of annealing is that the very heat that softens metal also makes it highly vulnerable to damage from the air. Nitrogen gas acts as a cost-effective, invisible shield, protecting the material's integrity and surface finish throughout the process.

The Core Problem: Metal's Reactivity at High Temperatures
To understand nitrogen's role, we must first understand the problem it solves. Annealing involves heating a metal to a specific temperature and then cooling it slowly to achieve desired properties.
What is Annealing?
Annealing is a heat treatment process primarily used to soften a metal, making it more ductile and less brittle. It also serves to relieve internal stresses that may have built up during prior manufacturing steps like cold working or machining.
The Threat of Oxidation
At elevated temperatures, most metals, particularly iron and steel, react readily with oxygen in the air. This reaction, known as oxidation, forms a brittle, flaky layer of metal oxide on the surface, commonly called "mill scale."
This scale is detrimental. It compromises the surface finish, can interfere with subsequent coating or plating operations, and represents a loss of material.
The Risk of Decarburization
For carbon steels, there is another significant risk: decarburization. At annealing temperatures, the carbon within the steel can react with oxygen or water vapor.
This reaction leaches carbon from the surface layer of the steel. The result is a soft, weak surface that no longer possesses the intended strength and wear resistance, which is a critical quality failure.
Nitrogen as the Protective Atmosphere
Using a controlled atmosphere is the solution to prevent these unwanted reactions. Nitrogen is the most common gas used for this purpose.
Creating an Inert Environment
Nitrogen (N₂) is a largely inert gas, meaning it does not readily react with other elements. Its atoms are held together by a very strong triple bond that is difficult to break at typical annealing temperatures.
By continuously purging the furnace chamber with nitrogen, the reactive oxygen (which makes up ~21% of air) is displaced. This starves the oxidation and decarburization reactions of the fuel they need to occur.
Why Nitrogen is the Ideal Choice
Nitrogen is selected over other gases for several key reasons:
- Abundance: It is the primary component of Earth's atmosphere (~78%), making it readily available and relatively inexpensive to produce.
- Inertness: As mentioned, its chemical stability makes it an effective shield that won't interfere with the metal's composition.
- Safety: It is non-flammable and non-toxic, making it safe to handle in industrial environments (with proper ventilation to prevent asphyxiation).
Beyond Pure Nitrogen: Protective Gas Mixtures
For applications requiring an even higher degree of surface purity, pure nitrogen can be mixed with other gases.
A common mixture is "forming gas," which is typically 95% nitrogen and 5% hydrogen. The small amount of hydrogen acts as a reducing agent, meaning it actively scavenges any residual oxygen and can even reverse minor surface oxidation, resulting in a "bright" finish.
Understanding the Trade-offs and Limitations
While highly effective, using a nitrogen atmosphere is not without considerations. Understanding these is key to successful implementation.
The Critical Role of Purity
The effectiveness of the nitrogen shield is directly tied to its purity. Any significant oxygen or moisture contamination in the nitrogen supply will compromise its protective qualities and can still lead to surface defects.
Cost vs. Benefit
Using a nitrogen atmosphere adds an operational cost for the gas itself and the required storage and delivery infrastructure. However, this cost is almost always justified by the prevention of scrap, rework, and product failure caused by oxidation and decarburization.
The Potential for Unwanted Nitriding
While nitrogen is inert with steels at most annealing temperatures, it can react with certain highly reactive metals (like titanium, magnesium, and aluminum) at high temperatures.
It can also react with some alloy steels at very high temperatures in a process called nitriding, which forms hard, brittle nitride compounds on the surface. This is generally undesirable in an annealing context, which aims to soften the material.
Making the Right Choice for Your Goal
The choice of atmosphere depends entirely on the material being treated and the desired final properties.
- If your primary focus is general-purpose annealing of carbon and low-alloy steels: High-purity nitrogen provides excellent, cost-effective protection against both scaling and decarburization.
- If your primary focus is achieving a bright, perfectly clean surface for plating or aesthetic purposes: A nitrogen-hydrogen blend (forming gas) is the superior choice for its active cleaning properties.
- If your primary focus is annealing highly reactive metals like titanium: A more truly inert gas like Argon may be necessary, as even nitrogen can react with the material at process temperatures.
Ultimately, controlling the furnace atmosphere is a fundamental pillar of modern heat treatment, ensuring the final product meets its precise engineering specifications.
Summary Table:
| Function | Benefit | Key Consideration |
|---|---|---|
| Displaces Oxygen | Prevents oxidation/scaling | Requires high-purity nitrogen |
| Creates Inert Atmosphere | Avoids decarburization in steels | Cost-effective for most metals |
| Can be mixed with Hydrogen | Achieves bright, clean finishes | May not suit reactive metals like titanium |
Optimize your annealing process with KINTEK's expertise in laboratory furnace atmospheres.
Whether you're working with carbon steels, alloys, or reactive metals, the right furnace atmosphere is critical for achieving your desired material properties. KINTCEL specializes in lab equipment and consumables, providing solutions that ensure precise temperature control and optimal gas environments for your heat treatment applications.
Contact our experts today to discuss how we can help you prevent surface defects, improve product quality, and select the perfect atmosphere solution for your specific annealing goals.
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- Why is an atmosphere sintering furnace used for post-annealing ZnO ceramics? Optimize Conductivity & Density
- Why is nitrogen used in furnaces? Key Benefits for High-Temperature Processes
- What are the potential dangers when working with inert gases? The Silent, Deadly Threat of Asphyxiation
- What advantages does a high-temperature atmosphere sintering furnace offer for UO2? Precision Fuel Densification
- What gases are used in a furnace? A Guide to Fuel vs. Process Atmospheres
- What role does a high-temperature atmosphere furnace play in Al0.5CoCrFeNi HEAs? Optimize Phase & Microstructure
- What are the primary advantages and disadvantages of using an oxygen probe? Optimize Your Atmosphere Control Strategy
- What is an inert gas atmosphere and for what applications is it used? Essential Guide for Heat Treatment & Lab Safety



















