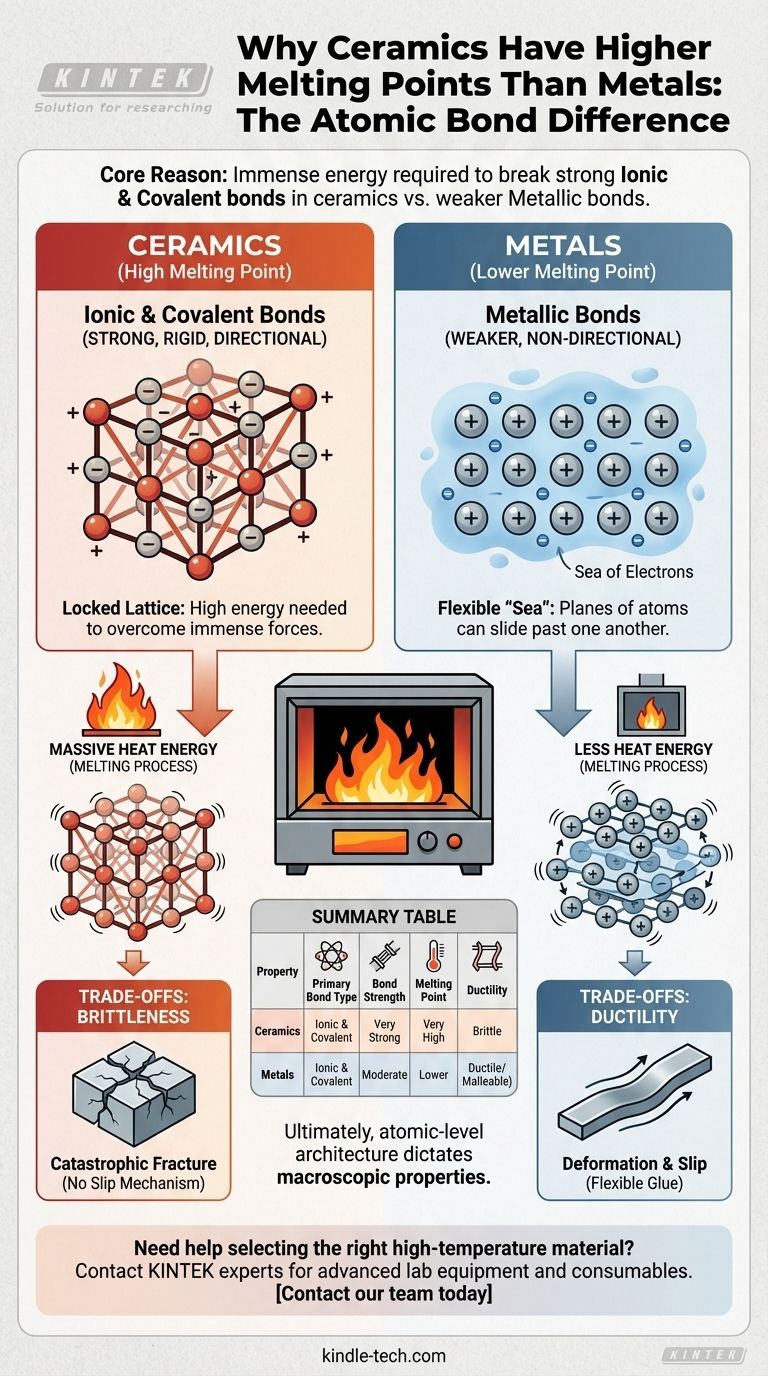
At its core, the immense difference in melting temperature between ceramics and most metals comes down to the fundamental nature of their atomic bonds. Ceramics are held together by extremely strong ionic and covalent bonds, which require a massive amount of energy to break, whereas metals are held together by weaker, non-directional metallic bonds.
The defining principle is simple: a material's melting point is a direct measure of the energy needed to break the bonds holding its atoms together. The powerful ionic and covalent bonds in ceramics create a rigid, stable structure that demands far more heat energy to dismantle than the flexible "sea of electrons" that defines metallic bonds.

The Defining Difference: Atomic Bonding
To understand thermal properties, we must first understand how atoms connect to one another. The type and strength of these connections are the primary factors that dictate a material's melting point.
Metallic Bonds: A "Sea" of Electrons
In metals, the outer electrons of the atoms are not tied to any single atom. Instead, they form a delocalized "sea" of electrons that flows freely around a fixed lattice of positive metal ions.
This arrangement creates a strong cohesive force, but the bonds are non-directional. This allows planes of atoms to slide past one another without catastrophic failure, which is why metals are ductile and malleable.
Ionic Bonds: A Powerful Attraction
Many ceramics are formed by ionic bonds, which occur between positively charged ions (cations) and negatively charged ions (anions). A classic example is a metal oxide.
The powerful electrostatic attraction between these opposite charges creates very strong, rigid, and directional bonds. This locks the ions into a highly stable crystalline lattice.
Covalent Bonds: The Shared Pair
Other ceramics, like silicon carbide, are defined by covalent bonds. Here, atoms share electrons to form stable electron pairs, creating exceptionally strong and highly directional links.
This type of bonding results in some of the hardest and most heat-resistant materials known, as breaking these shared pairs requires a tremendous amount of energy.
Ceramics: A Hybrid of Strength
Crucially, most advanced ceramics exhibit a mixture of ionic and covalent character. This combination produces an atomic structure with exceptionally high bond energy, creating a rigid framework that is incredibly resistant to the atomic vibrations induced by heat.
How Structure Dictates Thermal Stability
Bonding determines the atomic structure, and that structure determines how the material behaves when heated.
Melting: The Process of Breaking a Lattice
Melting is the process of supplying enough thermal energy to allow atoms or ions to break free from their fixed positions in the crystalline lattice. Heat is simply atomic vibration; the higher the temperature, the more violently the atoms vibrate.
Why Stronger Bonds Demand More Energy
To break the rigid, directionally-locked lattice of a ceramic, its atoms must vibrate with enough intensity to overcome the immense ionic and covalent forces holding them in place. This requires a very high temperature.
In contrast, the delocalized, non-directional nature of metallic bonds allows the lattice to be disrupted with significantly less thermal energy.
Understanding the Trade-offs
This high thermal stability in ceramics does not come without compromises. The nature of the bonding that provides strength also introduces significant limitations.
Strength Comes at a Cost: Brittleness
The same strong, directional bonds that give ceramics their high melting point also make them brittle. When a ceramic is subjected to stress, there is no easy mechanism for atoms to slide past one another.
Instead, the rigid bonds fracture catastrophically. Any small crack concentrates stress and propagates through the material with very little energy input.
The Advantage of Metals: Ductility
The "sea of electrons" in metals acts as a flexible glue. It allows atomic planes to slip and deform under stress without breaking the material's overall cohesion.
This property, known as ductility, is a direct trade-off for weaker bonds and, consequently, lower melting points.
Making the Right Choice for Your Application
Understanding these fundamental differences is critical for material selection in any engineering context.
- If your primary focus is high-temperature structural integrity: Ceramics are the unambiguous choice due to the exceptional thermal stability granted by their strong ionic and covalent bonds.
- If your primary focus is toughness, formability, and resistance to fracture: Metals are the superior option, as their non-directional metallic bonds allow for deformation rather than catastrophic failure.
- If you need a balance of properties: Consider advanced materials like ceramic-metal composites (cermets), which are engineered to combine the hardness of ceramics with the toughness of metals.
Ultimately, a material's macroscopic properties are a direct reflection of its atomic-level architecture.
Summary Table:
| Property | Ceramics | Metals |
|---|---|---|
| Primary Bond Type | Ionic & Covalent | Metallic |
| Bond Strength | Very Strong | Moderate |
| Melting Point | Very High | Lower |
| Ductility | Brittle | Ductile/Malleable |
Need help selecting the right high-temperature material for your application? At KINTEK, we specialize in providing advanced lab equipment and consumables for materials testing and research. Whether you're working with high-melting-point ceramics or ductile metals, our experts can help you choose the perfect solution for your laboratory's needs. Contact our team today to discuss how we can support your research and ensure optimal performance!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the pressureless sintering method? A Guide to Cost-Effective Material Densification
- What is the purpose of sintering in ceramics? Transforming Powder into Durable, High-Performance Parts
- At what temperature does ceramic melt? A Guide to Ceramic Heat Resistance
- What is the purpose of firing or sintering? To Transform Weak Powder into Strong, Dense Ceramics
- What are the synthesis methods of SiC? From Industrial Abrasives to High-Performance Electronics
- What function do alumina ceramic plates serve as supports in the preparation of molecular sieve membranes?
- How long does ceramic fiber last? Maximize Lifespan from Months to Decades
- What is the purpose of adding yttria to zirconia? Master High-Performance Ceramic Stability and Strength



















