Introduction to Chemical Vapor Deposition (CVD)
Definition and Function of CVD
Chemical Vapor Deposition (CVD) is a sophisticated technique used to create thin films by inducing chemical reactions on the surface of a substrate. This process involves the use of gas-phase compounds or monomers that contain the elements necessary for the formation of the thin film. The primary function of CVD is to facilitate the deposition of these elements onto the substrate, resulting in the creation of a uniform and high-quality thin film.
CVD is extensively employed in various scientific and industrial applications. One of its key uses is in the purification of substances, where it plays a crucial role in ensuring the purity of materials by removing impurities through controlled chemical reactions. Additionally, CVD is instrumental in the development of new crystalline structures, allowing researchers to explore and create novel materials with unique properties.
Furthermore, CVD is widely utilized for the precipitation of various inorganic thin-film materials. This capability makes it an essential tool in the fabrication of semiconductor devices, where the precise control over the deposition process is critical for the performance and reliability of electronic components. The versatility and precision of CVD make it a cornerstone technology in both research and industrial settings, driving advancements in materials science and electronics.
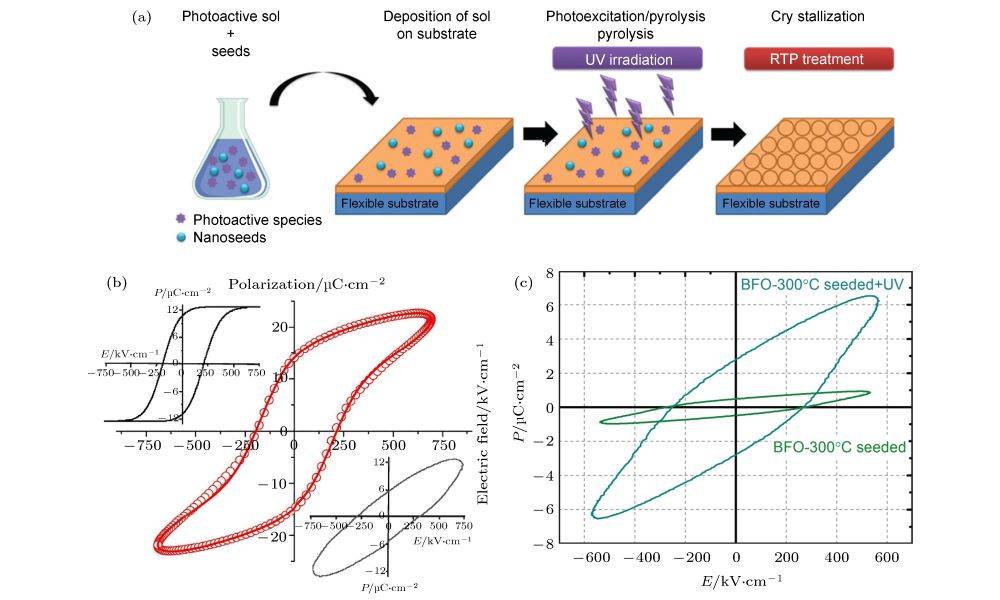
Applications in the Semiconductor Industry
Chemical Vapor Deposition (CVD) plays a pivotal role in the semiconductor industry, enabling the creation of advanced materials and structures essential for modern electronics. One of its primary applications is the deposition of polysilicon, a material widely used in the fabrication of microelectronic devices such as transistors and integrated circuits. Beyond polysilicon, CVD is instrumental in the synthesis of new amorphous materials, including phosphor-silica glass, borosilicate glass, silicon dioxide (SiO2), and silicon nitride (Si3N4). These materials are crucial for their insulating properties and their ability to form protective layers on semiconductor surfaces.
Furthermore, CVD processes are integral to the production of potential switching and storage memory materials, which are fundamental components in modern data storage technologies. The versatility of CVD in creating a wide array of thin films extends its applications beyond traditional semiconductors, finding utility in emerging technologies such as solar panels and advanced computer hardware. This broad applicability underscores the importance of CVD in driving innovations within the field of electrical engineering, promising significant advancements in the near future.
Electronic Specialty Gases in CVD
Functions of Electronic Specialty Gases
Electronic specialty gases play a multifaceted role in Chemical Vapor Deposition (CVD) processes, each type serving a distinct function crucial to the fabrication of semiconductor components. These gases can be broadly categorized into several key roles:
-
Feedstock/Doping Gases: These gases provide the essential elements needed for the formation of thin films. For instance, gases like silicon tetrachloride (SiCl4) and boron trichloride (BCl3) are used to introduce silicon and boron atoms, respectively, into the growing film.
-
Carrier Gases: Often inert, carrier gases such as argon (Ar) and nitrogen (N2) are used to transport reactive gases to the deposition chamber without altering their chemical composition. This ensures the precise delivery of the reactive gases to the substrate.
-
Reaction Atmosphere Gases: These gases create the necessary environment for the chemical reactions to occur. For example, hydrogen (H2) and oxygen (O2) are commonly used to facilitate the oxidation and reduction reactions that lead to the formation of various thin films.
-
Purge Gases: Purge gases like nitrogen (N2) are used to remove residual reactive gases and by-products from the deposition chamber. This step is critical to maintaining the purity of the deposition environment and ensuring the quality of the final product.
-
Purification Gases: Some gases, such as hydrogen fluoride (HF), are used for cleaning and etching processes, ensuring that the substrate surface is free from contaminants before the deposition process begins.
The precise control and management of these electronic specialty gases are essential for the successful fabrication of high-quality semiconductor components. Each gas type must be carefully selected and managed to meet the specific requirements of the CVD process, ensuring the integrity and performance of the final semiconductor device.

Types and Uses of Electronic Specialty Gases
Electronic specialty gases are integral to Chemical Vapor Deposition (CVD) processes, playing diverse roles in the fabrication of semiconductor components. These gases serve as feedstock, doping agents, carrier gases, reaction atmosphere gases, purge gases, and purification gases. Each gas type has specific applications within the CVD process, contributing to the precise and controlled deposition of thin films necessary for semiconductor manufacturing.
| Gas Type | Application in CVD Processes |
|---|---|
| Dichlorosilane (SiH2Cl2) | Used as a precursor for silicon deposition, essential for forming silicon-based thin films. |
| Silicon Tetrachloride (SiCl4) | Employed in the deposition of silicon dioxide (SiO2) layers. |
| Boron Trichloride (BCl3) | Acts as a dopant gas, introducing boron into silicon to modify its electrical properties. |
| Phosphine (PH3) | Functions as a dopant gas, adding phosphorus to silicon for n-type doping. |
| Arsine (AsH3) | Used as a dopant gas for introducing arsenic into silicon for n-type doping. |
| Ammonia (NH3) | Involved in the formation of nitride films, such as silicon nitride (Si3N4). |
| Methane (CH4) | Used in the deposition of carbon-based materials. |
| Hydrogen (H2) | Serves as a carrier gas and also aids in the reduction of metal precursors. |
| Argon (Ar) | Primarily used as a carrier gas, providing inert atmosphere during deposition. |
| Nitrogen (N2) | Acts as a carrier gas and is also used in the formation of nitride films. |
| Oxygen (O2) | Involved in the oxidation processes, essential for forming oxide layers. |
| Hydrogen Fluoride (HF) | Used for etching and cleaning processes within the CVD system. |
| Chlorine (Cl2) | Employed in etching processes to remove unwanted materials. |
These gases are meticulously selected and controlled to ensure the quality and consistency of the thin films deposited during CVD processes. Their precise use is critical for the successful fabrication of high-performance semiconductor devices.
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine Chemical Vapor Deposition Chamber System Equipment
- Laboratory CVD Boron Doped Diamond Materials
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine

















