Introduction to Three-Electrode System
Importance of Decoupling Electrode Properties
The three-electrode system stands as a pivotal tool in the realm of electrochemical research, particularly in the study of lithium batteries. This system is indispensable for dissecting and understanding the unique properties of individual electrochemical electrodes, a task that the traditional two-electrode setup falls short in achieving.
In the context of lithium batteries, the three-electrode configuration allows researchers to isolate and measure the electrochemical behavior of each electrode independently. This decoupling is crucial for identifying and addressing specific issues that may arise within the electrodes, such as potential imbalances or inefficiencies. By providing a clear and accurate picture of each electrode's performance, the three-electrode system enables more precise and targeted improvements in battery design and functionality.
Moreover, this system overcomes the inherent limitations of two-electrode setups, which often conflate the properties of the working and counter electrodes, leading to misleading or incomplete data. The three-electrode system's ability to separate these properties ensures that each electrode's contributions to the overall battery performance can be accurately assessed and optimized. This level of detail is essential for advancing the development of high-performance lithium batteries, driving innovations that can meet the increasing demands for energy storage solutions.
Characteristics of Reference Electrodes
Theoretical and Practical Requirements
For reference electrodes to function optimally in lithium batteries, they must possess several key characteristics. Firstly, they should be ideal unpolarized electrodes, meaning they maintain a stable potential under various conditions, ensuring accurate measurements. This stability is crucial for decoupling individual electrode properties, which is essential for understanding battery performance.
Secondly, these electrodes should exhibit a low reaction drive. This characteristic minimizes the potential for unwanted side reactions, thus preserving the integrity of the reference potential. A low reaction drive also enhances the electrode's ability to maintain a consistent potential, which is vital for accurate electrochemical testing.
Additionally, reference electrodes should have a large exchange current density. This property allows for rapid equilibration of charge transfer processes, ensuring that the electrode potential remains stable even under dynamic conditions. A high exchange current density is particularly important in batteries, where rapid charge and discharge cycles are common.
Lastly, good reversibility is essential. This means that the electrode should be able to undergo repeated oxidation and reduction processes without significant loss of performance. Good reversibility ensures long-term reliability and accuracy, making the reference electrode suitable for extensive use in both research and practical applications.
| Characteristic | Importance in Lithium Batteries |
|---|---|
| Ideal unpolarized | Stable potential, accurate measurements |
| Low reaction drive | Minimizes side reactions, preserves potential integrity |
| Large exchange current density | Rapid equilibration, stable potential under dynamic conditions |
| Good reversibility | Repeated use, long-term reliability and accuracy |
Specific Features for Lithium Batteries
When designing reference electrodes for lithium batteries, several specific features must be meticulously considered to ensure optimal performance and reliability. Miniaturization is a critical aspect, allowing for the integration of reference electrodes into compact battery systems without significantly altering the overall design. This ensures that the reference electrode does not impose additional space constraints, which is particularly important in applications where size and weight are critical factors.
Compatibility with the electrolyte is another essential feature. The reference electrode must interact seamlessly with the electrolyte to provide accurate potential readings. Any incompatibility can lead to inaccuracies in measurements and potentially degrade the battery's performance over time. Therefore, the material selection for the reference electrode must be carefully matched with the electrolyte composition to prevent unwanted reactions.
The absence of impurities in the reference electrode is crucial to maintain the integrity of the electrochemical measurements. Even trace amounts of impurities can introduce significant errors in potential readings, compromising the accuracy of the data. This necessitates rigorous quality control measures during the manufacturing process to ensure that the reference electrode is free from contaminants.
Lastly, a small temperature coefficient is vital for maintaining consistent performance across varying environmental conditions. Lithium batteries often operate in diverse temperature ranges, and the reference electrode must be able to provide stable and accurate readings regardless of the ambient temperature. This requires the use of materials and designs that minimize temperature-induced variations in potential.
In summary, the design of reference electrodes for lithium batteries must prioritize miniaturization, electrolyte compatibility, impurity-free composition, and a small temperature coefficient to ensure precise and reliable electrochemical measurements.
Design Considerations
Selection of Reference Electrode Type
When selecting a reference electrode for various battery types, it's essential to consider factors that minimize interference and ensure optimal stress distribution. The choice of reference electrode is influenced by several key features and considerations:
- Compatibility with the Sample: The reference electrode should not chemically interact with the battery's electrolyte or components, ensuring accurate and stable measurements.
- Stability of Potential: A stable potential is crucial for precise measurements. The reference electrode should maintain a constant potential, unaffected by external conditions or the battery's operational state.
- Response Time: Fast response times are necessary to maintain the efficiency of the analytical process, allowing for real-time data acquisition and analysis.
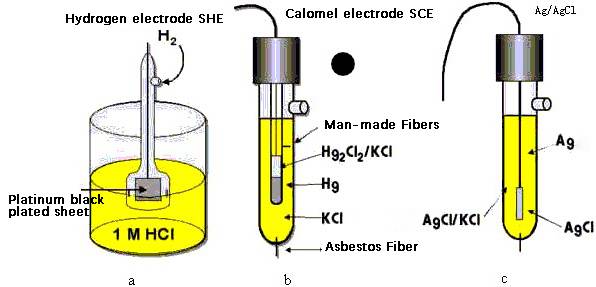
- Temperature Considerations: Different reference electrodes have varying temperature limits. For instance, the calomel electrode (SCE) is limited to 50°C. For higher temperature applications, alternative electrodes must be selected.
- Chemical Composition: The chemical composition of the sample can affect the electrode's material. Choosing the right material, such as glass, epoxy, or other specialized materials, is crucial to prevent degradation and ensure long-term stability.
Available Options
Several types of reference electrodes are available, each with its own set of advantages and limitations:
| Reference Electrode | Common Applications | Special Considerations |
|---|---|---|
| Saturated Calomel (Hg/HgCl) | General electrochemical testing | Contains mercury; unsuitable for food, beverage, or environmental studies |
| Ag/AgCl (wire or cartridge) | Most common type | Incompatible with samples containing Ag or Cl |
| Cu/CuSO4 | Specific applications requiring copper reference | Requires careful handling and maintenance |
| Hg/HgSO4 | High-temperature applications | Contains mercury; requires controlled disposal |
| Hg/HgO | Specialized applications | Contains mercury; requires controlled disposal |
Double Junction Electrodes
Double junction electrodes offer a customized solution by allowing the electrolyte in the lower chamber to be tailored to the sample's chemical composition. This customization is vital as it prevents interactions that could block the junction and lead to erratic readings.
By carefully considering these factors and options, the selection of a reference electrode can be optimized to ensure accurate, stable, and efficient performance in various battery applications.
Preparation Process
The preparation of reference electrodes for lithium batteries involves two primary methods: non-in situ and in situ preparation techniques. Each method has its unique advantages and challenges, significantly influencing the quality and performance of the final electrode.
Non-In Situ Preparation
Non-in situ methods typically involve the fabrication of the reference electrode outside the battery environment. This approach allows for meticulous control over the electrode's composition and structure, ensuring minimal impurities and optimal performance. However, the process can be time-consuming and may require specialized equipment to achieve the desired electrode quality.
In Situ Preparation
In contrast, in situ preparation techniques involve the creation of the reference electrode directly within the battery setup. This method is advantageous for real-time monitoring and can be more adaptable to varying experimental conditions. Despite its convenience, in situ preparation demands careful handling to prevent contamination and maintain electrode integrity.
Both methods play crucial roles in the development of high-quality reference electrodes, each catering to different research needs and experimental setups.
Reference Electrode Setting
Proper placement of reference electrodes is crucial for accurate potential detection, considering factors like proximity to study electrodes and electrolyte environment. The positioning of the reference electrode can significantly influence the measured potential, affecting the accuracy of the data collected during electrochemical testing.
When setting up a reference electrode, it is essential to ensure that it is placed as close as possible to the working electrode to minimize the potential difference due to the electrolyte resistance. This proximity helps in obtaining more precise measurements, especially in systems with high ionic resistance.
Additionally, the environment in which the reference electrode is placed must be carefully considered. The electrolyte composition, including its pH and ionic strength, can affect the reference electrode's performance. For instance, certain reference electrodes may not be suitable for use in highly acidic or alkaline environments without appropriate modifications.
To summarize, the optimal placement of a reference electrode involves a careful balance of proximity to the working electrode and compatibility with the electrolyte environment, ensuring accurate and reliable potential measurements.
Care and Maintenance
Choosing the Right Reference Electrode
Selecting the appropriate reference electrode and working conditions can significantly extend its service life. When choosing a reference electrode, several factors must be considered to ensure optimal performance and longevity.
Firstly, compatibility with the sample being measured is crucial. The reference electrode should not chemically interact with the sample or the electrolyte, as this can lead to inaccurate measurements and potential degradation of the electrode. For instance, certain chemicals can degrade the body material of the electrode, necessitating the selection of appropriate materials such as glass, epoxy, or other specialized materials to suit the application.

Another critical consideration is the stability of the potential provided by the reference electrode. A stable potential is essential for accurate measurements, ensuring that the reference electrode maintains a consistent and defined potential over time. This stability is determined by the electrolyte inside the electrode and the reference element used.
Temperature considerations are also vital. For example, the saturated calomel electrode (SCE) has a limited temperature range of up to 50°C. If the application requires use at higher temperatures, an alternative electrode must be selected. This is particularly important in environments where temperature fluctuations are common, as the reference electrode must be able to maintain its performance across a broad temperature spectrum.
The response time of the reference electrode is another key factor. A fast response time ensures efficiency in the analytical process, allowing for real-time data collection and analysis. This is especially important in applications where rapid changes in the sample or environment need to be monitored.
In some cases, it may be more practical or necessary to use separate sensing (half-cell) and reference electrodes. This is often the case when the different parts of the electrode are expected to have different lifespans or when specific applications require the use of separate electrodes. For instance, in certain high-precision measurements or in environments where the sample composition is highly variable, using separate electrodes can provide more accurate and reliable results.
A range of separate reference electrodes are available, each with its own advantages and limitations. Some of the most common reference systems include saturated calomel (Hg/HgCl), Ag/AgCl (wire or cartridge), Cu/CuSO4, Hg/HgSO4, and Hg/HgO. Ag/AgCl is the most common type of reference system, but if your sample is incompatible with Ag or Cl, a saturated calomel electrode (Hg/HgCl) might be a suitable alternative. However, calomel electrodes contain mercury, making them unsuitable for use in certain applications such as food, beverage, or environmental studies due to environmental implications.
Double junction electrodes offer another option, particularly in applications where compatibility with the sample is a concern. These electrodes have a lower chamber that contains an electrolyte that differs from the electrolyte in the top reference chamber. The chemical composition of the lower chamber electrolyte can be customized to match or be more compatible with the sample. This is important because the lower chamber electrolyte comes into contact with the sample via the junction, and any interaction between the electrolyte and sample can cause the junction to block, leading to erratic readings.
In conclusion, selecting the right reference electrode involves careful consideration of compatibility, stability, temperature range, response time, and specific application requirements. By taking these factors into account, you can ensure that your reference electrode performs reliably and extends its service life, providing accurate and consistent measurements in your application.
Regular Calibration and Maintenance
Regular calibration and maintenance of reference electrodes are essential to ensure the accuracy and reliability of their readings. This involves not only the periodic calibration of the electrode's potential but also the replacement of filling solutions. The filling solutions, which are integral to the electrode's operation, can degrade over time, leading to inaccuracies in potential measurements.
To maintain optimal performance, it is recommended to follow a strict maintenance schedule. This includes:
-
Regular Calibration: Conducting calibration at regular intervals to ensure that the electrode's potential remains within acceptable limits. This is typically done using standard reference solutions that are known to have stable and accurate potentials.
-
Replacement of Filling Solutions: Periodically replacing the filling solutions to prevent contamination and degradation. Contaminants can alter the solution's properties, affecting the electrode's performance. Degradation can lead to a drift in the electrode's potential, compromising its reliability.
| Maintenance Activity | Frequency | Purpose |
|---|---|---|
| Calibration | Every 3-6 months | Ensure potential accuracy |
| Replacement of Filling Solutions | Every 6-12 months | Prevent contamination and degradation, maintain solution integrity |
By adhering to these maintenance practices, the longevity and effectiveness of reference electrodes in lithium batteries can be significantly enhanced.
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
- Gold Disc Electrode
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
Related Articles
- Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
- AgAgCl Reference Electrode Working Principle and Applications
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- A Comprehensive Guide to Reference Electrodes
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
















