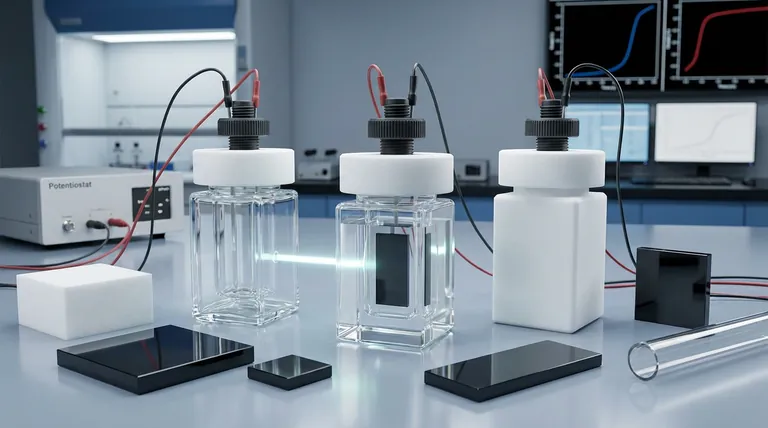
The Invisible Variable
In the high-stakes world of electrochemistry, we obsess over the variables we can control. We calculate voltages, adjust pH levels, and refine electrode surfaces with microscopic precision.
We focus entirely on the reaction. We rarely think about the wall that holds it.
This is a classic blind spot. As surgeon Atul Gawande might note about the operating room, system failures often occur not because of a lack of skill, but because of a failure in the structural environment. In the lab, the electrolytic cell body is that environment.
If the vessel reacts with your electrolyte, your data is noise. If the vessel blocks the light you need to measure, your sensors are blind.
The engineering challenge isn't finding a "perfect" material. In physics and materials science, "perfect" does not exist. There is only the correct trade-off for the specific stress tests of your experiment.
Here is how to navigate the architecture of containment.
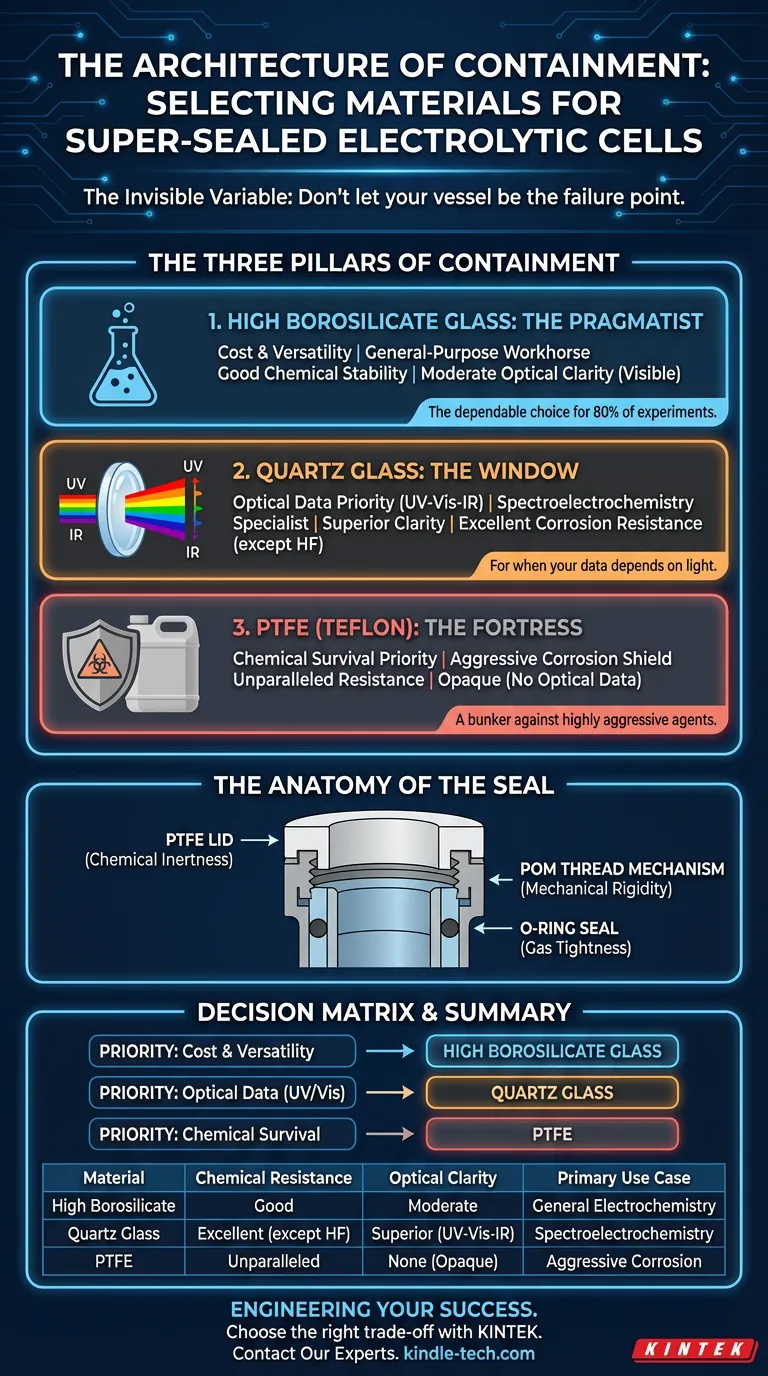
The Three Pillars of Containment
When designing a super-sealed electrolytic cell, we are generally choosing between three distinct material philosophies: High Borosilicate Glass, Quartz Glass, and Polytetrafluoroethylene (PTFE).
Each represents a different priority in the laboratory.
1. High Borosilicate Glass: The Pragmatist
For 80% of experiments, high borosilicate glass is the answer.
It is the dependable workhorse of the modern laboratory. It strikes a balance that is rare in materials science: it is chemically stable enough for most electrolytes, and it possesses high thermal resistance.
It is not invincible. But for general-purpose electrochemistry where extreme optical clarity or hyper-aggressive corrosion is not the primary concern, it is the most rational economic and technical choice. It does the job without complaint.
2. Quartz Glass: The Window
Sometimes, electricity isn't enough. You need to see.
In spectroelectrochemistry, the cell body is not just a container; it is a lens. Standard glass filters out ultraviolet light, effectively blinding your spectroscopic equipment.
Quartz glass is the optical specialist. Its atomic structure allows for excellent light transmittance across the full spectrum—from Ultraviolet (UV) to Visible to Infrared (IR).
It also boasts superior corrosion resistance against strong acids and weak bases. However, it has a specific Achilles heel: hydrofluoric acid. Aside from that vulnerability, it is the clear choice when your data depends on light.
3. PTFE (Teflon): The Fortress
There are environments where glass—no matter how strong—simply cannot survive.
When dealing with highly aggressive chemical agents, you need a material that acts as a shield. PTFE (Polytetrafluoroethylene) provides unparalleled corrosion resistance. It is the stoic of materials; it refuses to interact with almost anything.
The trade-off is visibility. PTFE is opaque. You gain ultimate chemical security, but you lose the ability to perform optical measurements through the cell body. It is a bunker, not a greenhouse.
The Anatomy of the Seal
A robust material is useless if the system leaks.
The term "super-sealed" implies a systemic approach to gas tightness. While the body varies (Glass, Quartz, or PTFE), the supporting infrastructure usually relies on polymers known for their mechanical strength and inertness.
- The Lids: Almost exclusively PTFE to maintain chemical inertness at the headspace.
- The Mechanism: External thread structures often utilize POM (Polyoxymethylene). POM provides the mechanical rigidity required to screw caps down tightly, ensuring firm, even pressure on the O-rings without deforming.
The Decision Matrix
Making the right choice requires honest anticipation of your experimental conditions.
If you optimize for everything, you optimize for nothing. You must choose your priority:
- Priority: Cost & Versatility $\rightarrow$ Choose High Borosilicate Glass.
- Priority: Optical Data (UV/Vis) $\rightarrow$ Choose Quartz Glass.
- Priority: Chemical Survival $\rightarrow$ Choose PTFE.
Summary of Material Properties
| Material | Chemical Resistance | Optical Clarity | Primary Use Case |
|---|---|---|---|
| High Borosilicate | Good | Moderate (Visible only) | General Electrochemistry |
| Quartz Glass | Excellent (except HF) | Superior (UV-Vis-IR) | Spectroelectrochemistry |
| PTFE | Unparalleled | None (Opaque) | Aggressive Corrosion Studies |
Engineering Your Success
The integrity of your data begins with the integrity of your equipment. A compromised vessel introduces variables that no amount of data processing can remove.
At KINTEK, we understand that lab equipment is not a commodity; it is a component of the scientific method. We provide super-sealed electrolytic cells in all three primary materials, engineered with precision sealing mechanisms to ensure your environment remains controlled.
Whether you need the transparency of quartz or the chemical armor of PTFE, we help you make the right trade-off.
Contact Our Experts to discuss your experimental parameters and secure the precise vessel your research demands.
Visual Guide
Related Products
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Custom PTFE Teflon Parts Manufacturer for PTFE Ball Valve Seat
- Custom PTFE Teflon Parts Manufacturer for Acid and Alkali Resistant Chemical Powder Material Scoops
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- Laboratory CVD Boron Doped Diamond Materials
Related Articles
- The Silent Interface: Mastery Over Electrode Decay
- The Silent Geometry of Voltage: Respecting the Limits of Electrolysis
- All About ACTIVATED CARBON THERMAL REGENERATION
- Preparation of Graphene by Chemical Vapor Deposition (CVD)
- Preparation and Transfer Technology of Graphene by Chemical Vapor Deposition