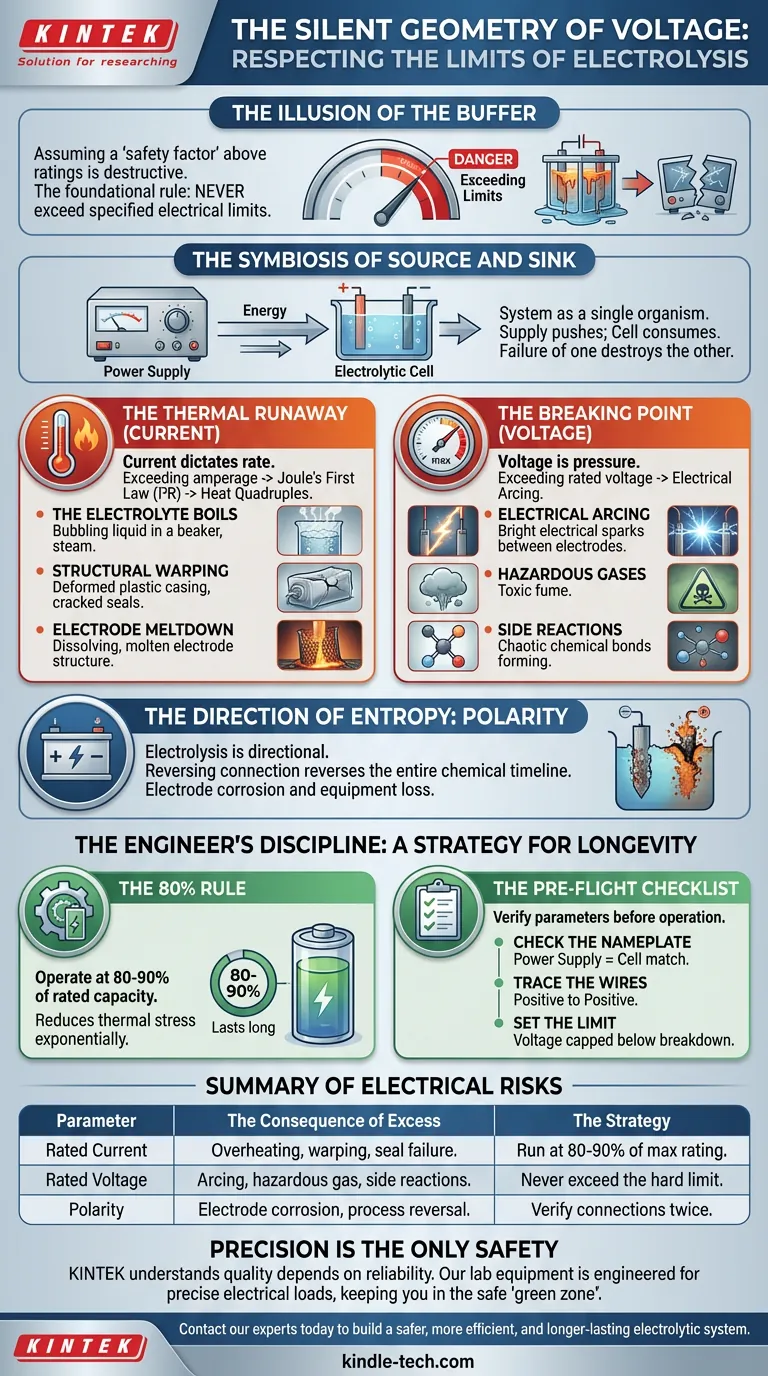
The Illusion of the Buffer
In engineering and laboratory sciences, we often operate with an implicit belief in the "safety factor." We assume that a bridge can hold more than its weight limit, or that a beaker can withstand a few degrees above its rating.
We assume the red line on the dial is a suggestion, not a cliff.
In the world of electrolysis, this mindset is not just wrong; it is destructive. The foundational rule of operating an electrolytic cell is absolute: never exceed the specified electrical limits of your components.
This is not about bureaucratic compliance. It is about physics.
The ratings for current (amperage) and voltage are the boundaries where chemistry functions predictable. Step outside them, and you enter a chaotic state where equipment melts, electrolytes break down, and safety evaporates.
The Symbiosis of Source and Sink
To understand the risk, we must look at the system as a single organism.
The power supply and the electrolytic cell are symbiotic. The power supply pushes energy; the cell consumes it. If the cell demands more than the supply can give, the supply burns out. If the supply forces more than the cell can take, the cell degrades.
There is no isolation here. The failure of one component almost always guarantees the destruction of the other.
The Thermal Runaway (Current)
Current is the measure of flow. In electrolysis, it dictates the rate of reaction. It is tempting to turn the dial up to speed the process down.
But current generates heat.
Every conductor has resistance. According to Joule’s First Law ($I^2R$), doubling the current doesn't just double the heat—it quadruples it.
When you exceed the rated amperage:
- The Electrolyte Boils: Thermal stress alters the chemical composition.
- Structural Warping: Seals fail, and plastic casings deform.
- Electrode Meltdown: The physical structure of the electrode cannot dissipate the energy fast enough.
The Breaking Point (Voltage)
If current is flow, voltage is pressure.
Exceeding the rated voltage is akin to over-pressurizing a pipe. The energy must go somewhere. In an electrolytic cell, excess voltage often results in electrical arcing.
It forces the electrolyte to break down in ways you did not intend. This creates side reactions, producing hazardous gases or contaminating your pure output. You are no longer doing chemistry; you are creating chaos.
The Direction of Entropy: Polarity
There is a third variable, often overlooked in the rush to begin: Polarity.
Electrolysis is strictly directional. The anode (positive) and cathode (negative) are chemically distinct environments.
Reversing the connection is not a minor error. It reverses the entire chemical timeline. An electrode designed to be inert may suddenly begin to corrode and dissolve into your solution.
You don't just lose time. You lose the equipment.
The Engineer’s Discipline: A Strategy for Longevity
How do we prevent this? By shifting our mindset from "maximum capacity" to "optimal reliability."
The most experienced operators do not run their machines at the red line. They understand that longevity is a function of restraint.
The 80% Rule
If your goal is equipment longevity, never run your system at 100% of its rated capacity.
Operate at 80-90%.
This 10-20% buffer reduces thermal stress exponentially. It is the difference between a cell that lasts a month and one that lasts a year.
The Pre-Flight Checklist
Treat your electrolytic setup like an aircraft. Before the switch is flipped, the parameters must be verified.
- Check the Nameplate: Does the power supply match the cell?
- Trace the Wires: Is positive connected to positive?
- Set the Limit: Is the voltage capped below the breakdown threshold?
Summary of Electrical Risks
| Parameter | The Consequence of Excess | The Strategy |
|---|---|---|
| Rated Current | Overheating, warping, seal failure. | Run at 80-90% of max rating. |
| Rated Voltage | Arcing, hazardous gas, side reactions. | Never exceed the hard limit. |
| Polarity | Electrode corrosion, process reversal. | Verify connections twice. |
Precision is the Only Safety
In electrolysis, safety is not a separate feature. It is the natural result of precision.
At KINTEK, we understand that the quality of your results depends on the reliability of your tools. Our lab equipment and consumables are engineered to handle precise electrical loads, giving you the control you need to stay within the safe "green zone" of operation.
Do not leave your chemical processes to chance or inferior components.
Contact our experts today to discuss how KINTEK can help you build a safer, more efficient, and longer-lasting electrolytic system.
Visual Guide
Related Products
- Electrode Polishing Material for Electrochemical Experiments
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Copper Foam
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
Related Articles
- Electrochemical Electrodes in Chemical Analysis
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
- A Beginner's Guide to Understanding Reference Electrodes in Electrochemistry
- Electrode Materials for Rotating Ring-Disk Electrodes
- Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies




















