The Architecture of Fragility
There is a specific kind of romance in spectroelectrochemistry. You are attempting to capture the fleeting moments of a chemical reaction using the precise physics of light, all happening within a vessel that fits in the palm of your hand.
But this romance is housed in glass or quartz.
The side-window optical electrolytic cell is a paradox. It must be transparent enough to allow light to pass without distortion, yet strong enough to contain corrosive electrolytes and electrical currents. It is a system where physics, chemistry, and engineering collide.
If you treat this equipment as merely a beaker with a window, you will fail.
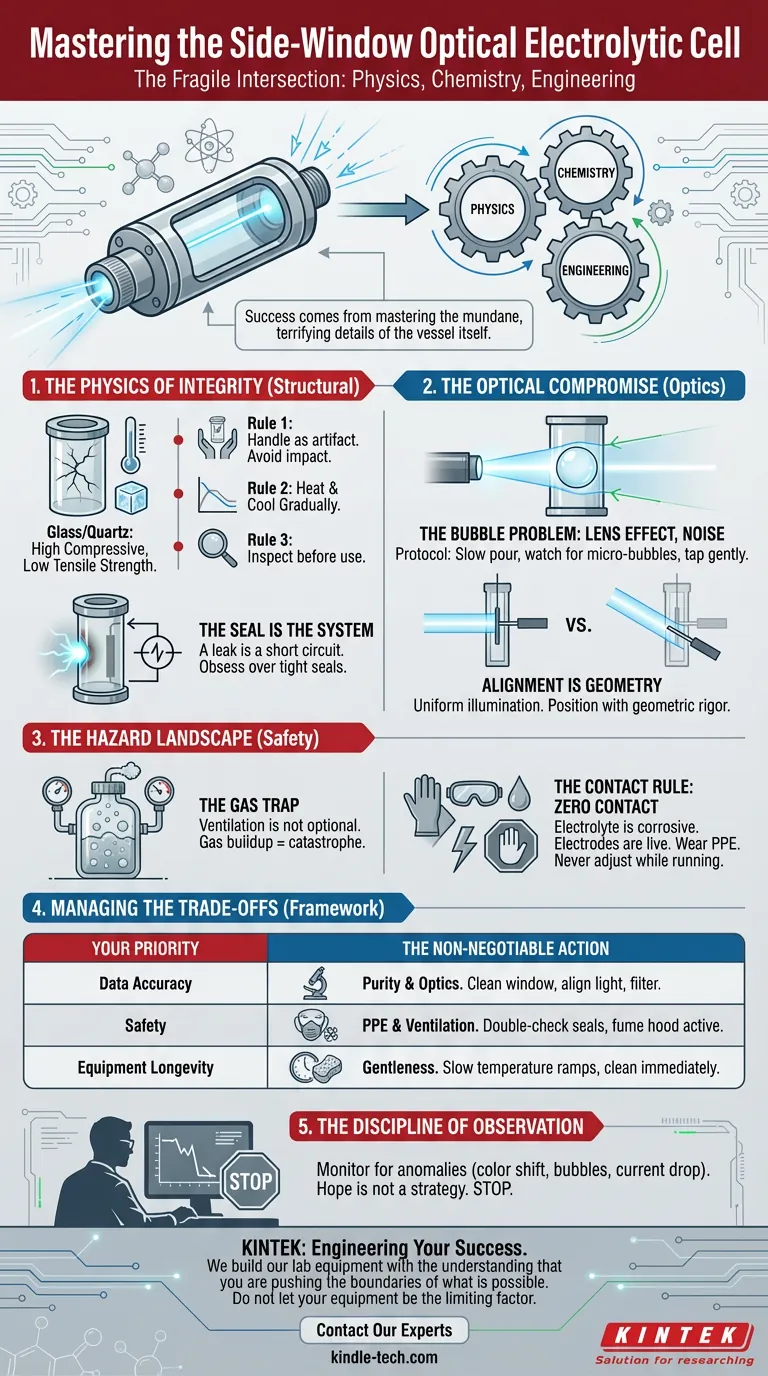
Success in this field doesn't come from a breakthrough hypothesis alone. It comes from mastering the mundane, terrifying details of the vessel itself. Here is how to navigate the intersection of fragility and precision.
The Physics of Integrity
The first failure point is almost always structural.
Glass and quartz are materials of high compressive strength but low tensile strength. They are unforgiving. A cell does not wear out; it shatters.
The Thermal Shock Trap
We often forget that temperature is a physical force. When heating a cell, you are expanding the material. If that expansion happens unevenly, the stress fractures the lattice.
- Rule 1: Handle the cell as if it were an artifact. Avoid impact with hard surfaces.
- Rule 2: Respect thermodynamics. Heat gradually. Cool gradually.
- Rule 3: Never assume the vessel is sound. Inspect it before the experiment begins.
The Seal is the System
In standard chemistry, a leak is a mess. In electrochemistry, a leak is a short circuit.
The interface between your electrode, the window, and the cell body is the weakest link. If the electrolyte escapes, it compromises your data first, and your safety second.
You must obsess over the seals. Ensure components fit tightly. A perfect seal is not a "nice to have"—it is the boundary condition that allows the experiment to exist.
The Optical Compromise
The defining feature of this cell—the window—is also its most temperamental variable.
You are trying to measure light absorption or emission. Anything that gets in the way of that light is noise. In this context, noise isn't just static; it is false data.
The Bubble Problem
There is a psychology to pouring liquid. We want to be done quickly.
But pouring electrolyte quickly entrains air. In a side-window cell, a single bubble adhering to the quartz window or the working electrode acts as a lens. It refracts your light source and insulates the electrode surface.
The protocol must be slow:
- Pour the electrolyte gently through the designated opening.
- Watch for micro-bubbles.
- If they appear, gently tap the cell. Do not proceed until the optical path is clear.
Alignment is Geometry
You cannot "eyeball" the light source.
The beam must illuminate the working electrode uniformly. If the alignment is off by a few degrees, you are measuring the solution, not the reaction at the interface. Position your light source with geometric rigor.
The Hazard Landscape
We grow comfortable in the lab. We forget that an electrolytic cell is an active hazard zone.
You are running current through a chemical solution. This generates heat and, frequently, gases.
The Gas Trap
Electrolysis produces gas. In a stagnant, sealed environment, pressure builds. If the gas is flammable (like hydrogen) or toxic, a crack in the glass becomes a catastrophe.
Ventilation is not optional. You must ensure that the gases produced have somewhere to go, and that place is away from your lungs and away from ignition sources.
The Contact Rule
The electrolyte is often corrosive. The electrodes are live.
The rule is simple: Zero Contact.
Treat the active setup as if it were radioactive. Wear gloves. Wear goggles. Never adjust the cell while the current is running. The trade-off for data should never be your own biology.
Managing the Trade-offs
Every experiment is a negotiation between competing priorities. You cannot maximize everything at once. You must decide what matters most for your specific run.
Here is a framework for decision-making:
| Your Priority | The Non-Negotiable Action |
|---|---|
| Data Accuracy | Purity & Optics. Obsess over cleaning the window and aligning the light source. Filter the electrolyte to remove particulates. |
| Safety | PPE & Ventilation. Double-check the seals. Ensure the fume hood is active. Wear splash-proof goggles. |
| Equipment Longevity | Gentleness. Slow temperature ramps. careful handling. Cleaning immediately after use to prevent corrosion. |
The Discipline of Observation
The final component is you.
An automated system can record data, but it cannot understand context. You must monitor the cell. Look for the physical changes—the color shift, the bubble formation, the sudden drop in current.
If you see an anomaly, stop. Do not hope it will correct itself. Hope is not a strategy in laboratory science.
Engineering Your Success
The difference between a failed experiment and a breakthrough often lies in the quality of the tools and the discipline of the operator. At KINTEK, we understand the engineer's romance. We know that the glass must be perfect, the seals must be tight, and the optics must be flawless.
We build our lab equipment with the understanding that you are pushing the boundaries of what is possible. We handle the hardware so you can handle the science.
Do not let your equipment be the limiting factor.
Contact Our Experts today to find the precise side-window electrolytic cell that fits your research needs.
Visual Guide
Related Products
- Side Window Optical Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Related Articles
- Understanding Electrodes and Electrochemical Cells
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
- Advanced Electrolytic Cell Techniques for Cutting-Edge Lab Research
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Escaping the Black Box: The Architecture of Insight in Electrochemistry




















