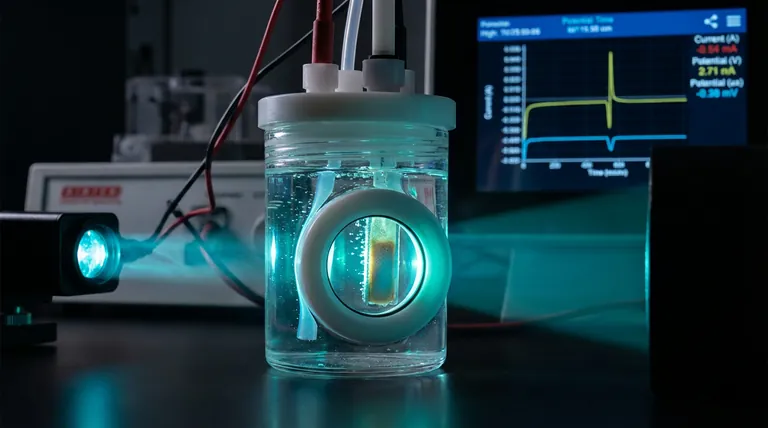
The Limits of Blind Data
In standard electrochemistry, we often work in the dark.
You set up your cell. You apply a voltage. You watch the numbers scroll across a screen—current, potential, impedance. You get a graph that tells you something happened.
But graphs are abstractions. They are shadows of the reality occurring on the electrode surface.
Did the film degrade? Did the color shift? Was the gas evolution uniform?
In a standard opaque setup, you are guessing. You are treating the reaction as a "black box," feeding inputs and analyzing outputs without witnessing the process.
To truly understand the mechanism, you need to add a new sense to your data: Sight.
This is the engineering philosophy behind the Side-Window Optical Electrolytic Cell. It is not just a container; it is an instrument designed to bridge the gap between electrical data and physical reality.
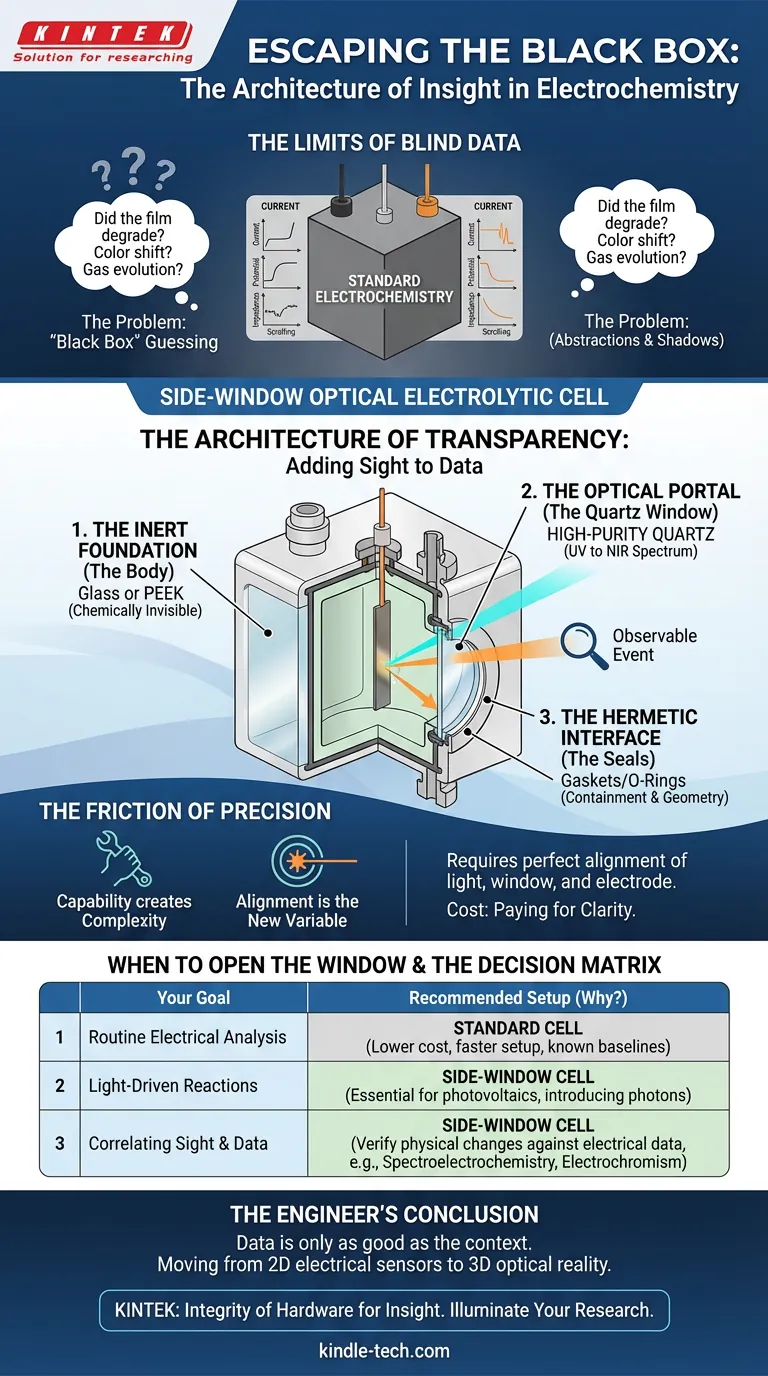
The Architecture of Transparency
A side-window cell is a specialized vessel designed for experiments that merge optical analysis with electrochemistry.
It is built on a simple premise with complex execution: allow a researcher to shine light onto an electrode while simultaneously measuring the electrical response.
It transforms the experiment from a blind measurement into an observable event. To achieve this, the hardware requires three specific engineering choices.
1. The Inert Foundation (The Body)
The cell body is the stage. It holds the electrolyte.
It is typically machined from Glass or PEEK (polyether ether ketone). The choice of material is not aesthetic; it is defensive. The body must remain chemically invisible, refusing to react with the solution even under aggressive potentials.
2. The Optical Portal (The Quartz Window)
This is the defining feature. Standard glass blocks UV light.
Side-window cells utilize high-purity quartz. Quartz is transparent across a broad spectrum, from Ultraviolet (UV) to visible and Near-Infrared (NIR).
This allows the window to act as a precise interface. It lets a controlled beam of light enter the "black box" and strike the working electrode without distortion or absorption.
3. The Hermetic Interface (The Seals)
The most common point of failure in any pressurized or fluid system is the joint.
The electrode ports use gaskets or O-rings to create a hermetic seal. This serves two purposes:
- Containment: It prevents electrolyte leakage.
- Geometry: It locks the electrodes in a fixed, reproducible position. In optics, geometry is everything.
The Friction of Precision
There is a psychological trade-off in engineering: Capability creates complexity.
A beaker is easy to use. A side-window cell is a precision instrument. Using one requires a shift in mindset.
Alignment is the new variable. In a standard cell, you just drop the electrodes in. In a side-window cell, the light source, the quartz window, and the working electrode must be perfectly aligned. If the angle is off by a fraction of a degree, your light misses the target, or the refraction skews your data.
The Optical Path. You must account for the electrolyte itself. The fluid between the window and the electrode can scatter or absorb light. The distance is no longer arbitrary; it is a variable in your equation.
The Cost. High-purity quartz and precision machining cost more than standard glass. You are paying for the privilege of clarity.
When to Open the Window
Not every experiment needs this level of scrutiny.
If you are running routine Cyclic Voltammetry (CV) to check a known standard, this tool is overkill. Complexity without purpose is just waste.
However, if you are working at the frontier of materials science, "seeing" is not optional.
You need a side-window cell if:
- Solar Fuels: You are testing materials for photovoltaics and need to drive the reaction with light.
- Spectroelectrochemistry: You need to correlate a spike in current with a change in absorbance or fluorescence.
- Electrochromism: You are studying materials that change color under potential.
The Decision Matrix
| Your Goal | Recommended Setup | Why? |
|---|---|---|
| Routine Electrical Analysis | Standard Cell | Lower cost, faster setup, sufficient for known baselines. |
| Light-Driven Reactions | Side-Window Cell | Essential. You must introduce photons to the electrode surface. |
| Correlating Sight & Data | Side-Window Cell | Required to verify physical changes (color/bubbles) against electrical data. |
The Engineer’s Conclusion
Data is only as good as the context surrounding it.
When you rely solely on electrical sensors, you are seeing a reaction in 2D. Adding an optical window renders it in 3D. It allows you to confirm that the spike in your graph actually correlates to the physical degradation you suspected.
At KINTEK, we understand that the quality of your research often rests on the integrity of your hardware. We build side-window optical electrolytic cells for researchers who refuse to accept the "black box."
If you are ready to illuminate your research and stop guessing at what is happening inside your reactor, we can help you configure the precise alignment you need.
Visual Guide
Related Products
- Side Window Optical Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
Related Articles
- Advanced Electrolytic Cell Techniques for Cutting-Edge Lab Research
- The Fragile Intersection: Mastering the Side-Window Optical Electrolytic Cell
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
- Understanding Electrodes and Electrochemical Cells