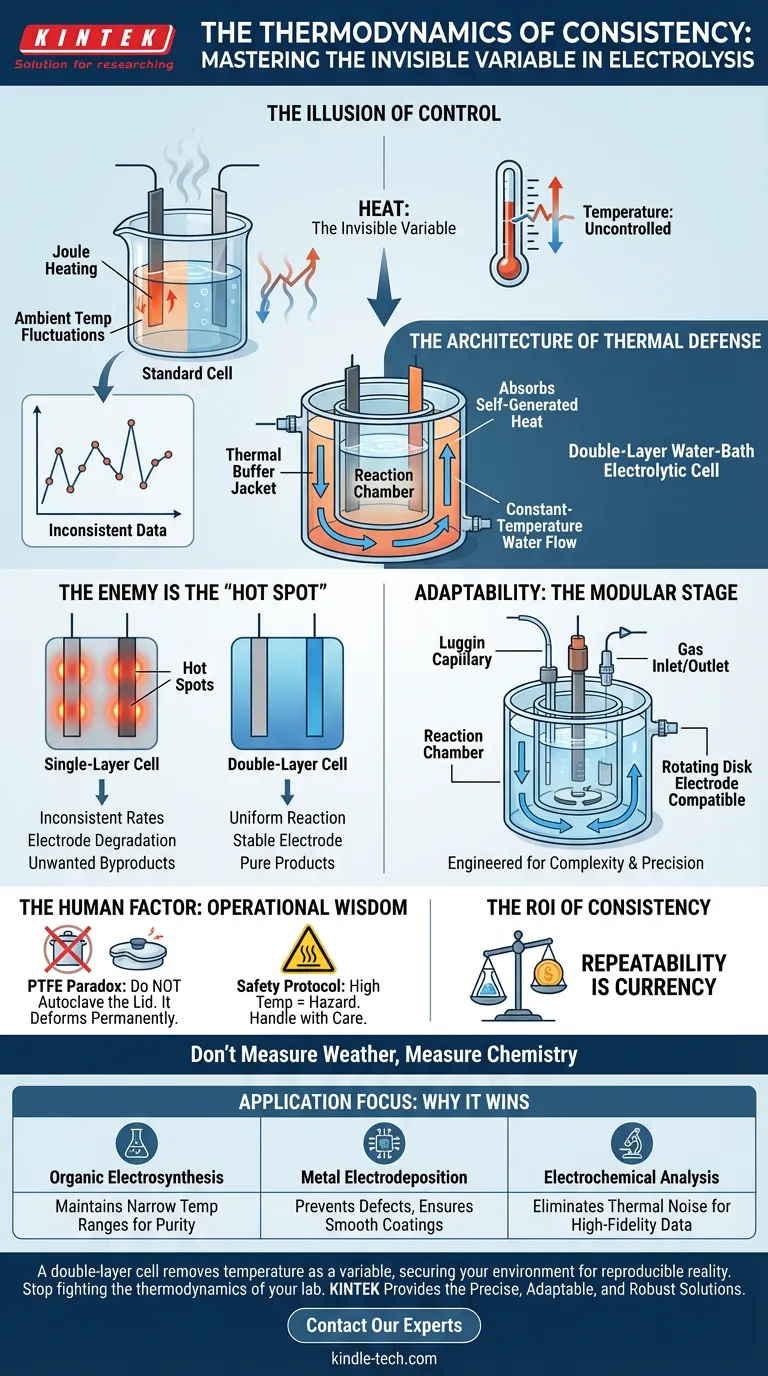
The Illusion of Control
In the laboratory, we obsess over variables we can see. We meticulously calibrate voltage. We measure current down to the microampere. We time reactions to the second.
But there is an invisible variable that often undermines the most rigorous electrochemical experiments: Heat.
Electrolysis is not a static process. It generates energy. As current flows, resistance creates heat (Joule heating). Simultaneously, the ambient temperature of your lab fluctuates throughout the day.
If you cannot control the temperature, you cannot trust the data.
This is where the double-layer water-bath electrolytic cell ceases to be a simple glass container and becomes a necessary instrument of precision. It is an engineering solution to the problem of entropy.
The Architecture of Thermal Defense
The double-layer cell addresses a fundamental truth of chemistry: reaction rates, product selectivity, and electrode stability are slaves to temperature.
The Thermal Buffer
The cell operates on a simple but profound design philosophy. It consists of two nested containers:
- The Inner Chamber: Where your reaction occurs.
- The Outer Jacket: A protective shell filled with circulating liquid (usually water) from a constant-temperature bath.
This jacket acts as a thermal firewall. It isolates your experiment from the chaotic temperature shifts of the room. More importantly, it absorbs the self-generated heat from the electrolysis, wicking it away before it can alter the reaction kinetics.
The Enemy is the "Hot Spot"
Temperature control is not just about the average reading on a thermometer. It is about uniformity.
In a standard single-layer cell, heat accumulates unevenly. This creates "hot spots" on the electrode surface.
Hot spots are dangerous. They cause local acceleration of the reaction, leading to:
- Inconsistent reaction rates.
- Degradation of the electrode.
- Formation of unwanted byproducts.
The circulating water in a double-layer cell ensures that every square millimeter of the electrode experiences the exact same thermal environment. It forces the system into equilibrium.
Adaptability: The Modular Stage
While thermal stability is the foundation, utility is the structure. A piece of lab equipment must be as flexible as the questions you are asking.
The best double-layer cells are designed as modular platforms for the three-electrode system—the gold standard of electrochemical analysis.
Engineered for Complexity
Modern research rarely relies on a simple anode and cathode. The cell design accommodates this complexity through configurable ports:
- The Luggin Capillary: Allows the reference electrode to sit close to the working electrode, minimizing iR drop (voltage loss due to resistance).
- Gas Management: Dedicated inlets for purging oxygen or blanketing the electrolyte with inert gas, essential for sensitive organic electrosynthesis.
- Dynamic Elements: Compatibility with rotating disk electrodes for hydrodynamic studies.
Whether you are performing metal electrodeposition or delicate organic synthesis, the cell adapts to the physics of the experiment.
The Human Factor: Operational Wisdom
Even the most robust system has limits. The double-layer cell is a precision tool, not a blunt instrument. Its longevity depends on respecting the materials.
The PTFE Paradox Glass is resilient; PTFE (Teflon) is stubborn. While the glass body can handle the autoclave, the PTFE sealing lid cannot.
PTFE expands when heated. If you autoclave the lid, it deforms. When it cools, it will not return to its original shape. The seal is broken, and the cell is compromised.
The Safety Protocol Thermal control implies heat. When running high-temperature experiments, the glass and tubing become hazardous to the touch. The system protects the reaction, but the operator must protect themselves.
The ROI of Consistency
Why invest in a double-layer system? Because in science, repeatability is the only currency that matters.
If your results fluctuate because the AC unit in the lab turned off, you are not measuring chemistry; you are measuring the weather.
| Application Focus | Why the Double-Layer Cell Wins |
|---|---|
| Organic Electrosynthesis | Maintains specific narrow temp ranges to ensure product purity. |
| Metal Electrodeposition | Prevents defects by stopping hot spots; ensures smooth coatings. |
| Electrochemical Analysis | Eliminates thermal noise, providing high-fidelity data. |
Conclusion
The difference between a failed experiment and a breakthrough often lies in the variables we choose to ignore.
A double-layer water-bath electrolytic cell removes the variable of temperature from the equation. It allows you to focus on the chemistry, knowing that the environment is secure.
At KINTEK, we understand that your equipment is the silent partner in your research. We provide the precise, adaptable, and robust electrolytic cells required to turn theoretical chemistry into reproducible reality.
Stop fighting the thermodynamics of your lab. Contact Our Experts
Visual Guide
Related Products
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Related Articles
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Glass Heart of the Experiment: Precision Through Systematic Care
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
- The Unseen Variable: Mastering the Electrolytic Cell Inspection




















