Yes, graphite is a highly effective conductor of both electricity and heat. Unlike most non-metals, graphite's unique atomic structure allows it to behave much like a metal in its ability to conduct. This is why it has very low electrical resistance and excellent resistance to thermal shock, making it a critical material in many high-performance applications.
The key to understanding graphite is recognizing its dual nature. It is a non-metal that conducts electricity and heat exceptionally well, but almost exclusively along its two-dimensional layers, a property that stems directly from its unique arrangement of carbon atoms.

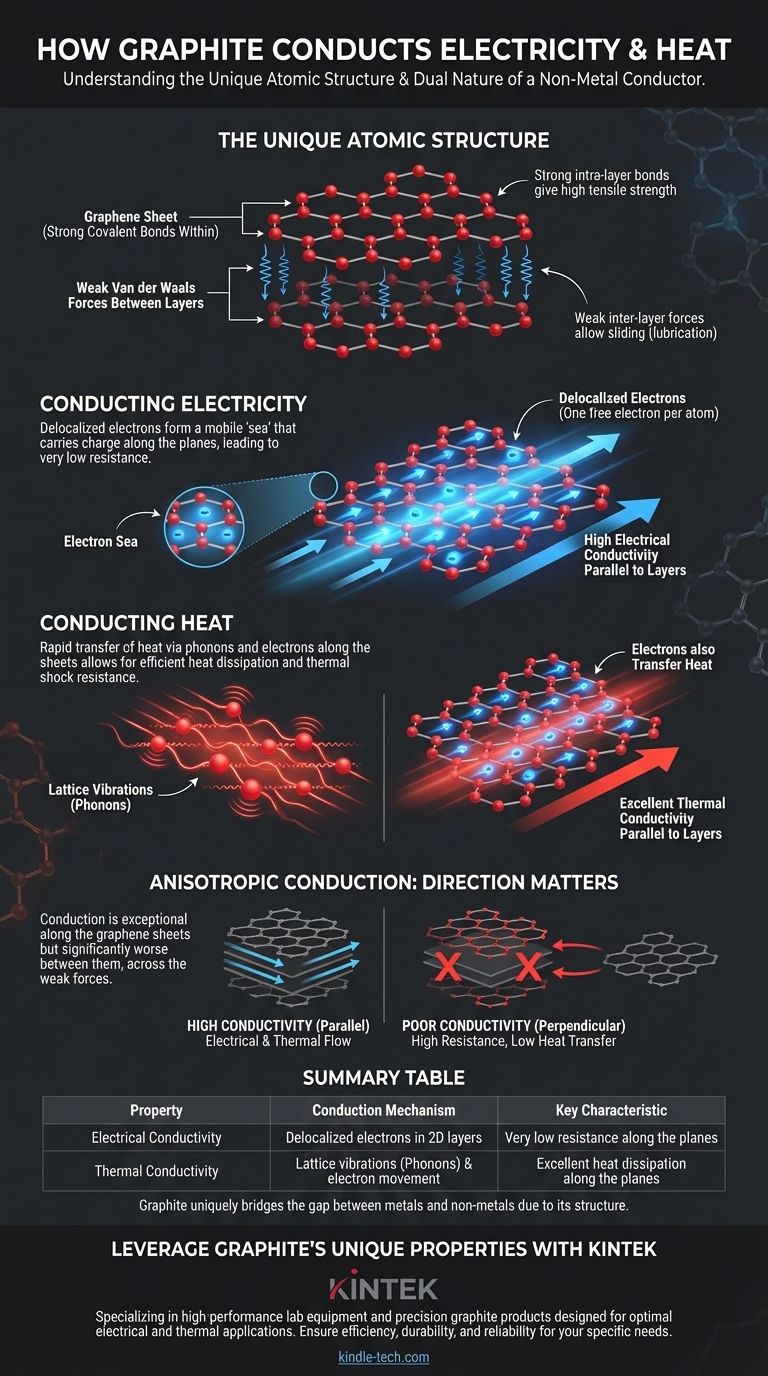
The Unique Atomic Structure of Graphite
To grasp why graphite conducts, we must first look at its fundamental structure. It is an allotrope of carbon, meaning it's made of the same atoms as diamond, but arranged in a profoundly different way.
Strong Bonds Within Layers
Graphite is composed of countless layers of carbon atoms. Within each layer, every carbon atom is bonded to three other carbon atoms in a hexagonal lattice, forming a flat, sheet-like structure often called a graphene sheet.
These covalent bonds are incredibly strong, giving the individual layers of graphite immense tensile strength and stability.
Weak Bonds Between Layers
While the atoms within a layer are strongly bonded, the layers themselves are held together by much weaker forces known as van der Waals forces.
These weak connections allow the layers to slide past one another easily, which is what gives graphite its characteristic softness and lubricating properties.
How Graphite Conducts Electricity
Graphite's ability to conduct electricity is a direct result of its bonding structure within the carbon layers.
The Role of Delocalized Electrons
Each carbon atom has four outer electrons available for bonding. In graphite, only three of these electrons are used to form the strong covalent bonds with neighboring atoms in the hexagonal sheet.
This leaves one electron per atom—the fourth one—unattached. This electron becomes delocalized, meaning it is free to move anywhere within its two-dimensional layer.
An "Electron Sea" in Two Dimensions
These free-moving electrons form a mobile "sea" of charge. When a voltage is applied, these delocalized electrons can flow easily along the layers, creating a powerful electrical current.
This mechanism is why graphite has such low electrical resistance, a property mentioned in high-performance materials like isostatic graphite.
How Graphite Conducts Heat
The same structural features that allow for electrical conductivity also make graphite an excellent thermal conductor.
Lattice Vibrations (Phonons)
Heat energy is primarily transferred through a material via vibrations in its atomic lattice. The strong covalent bonds within graphite's layers allow these vibrations, known as phonons, to travel very quickly and efficiently across the sheet.
This rapid transfer of vibrational energy results in high thermal conductivity.
The Dual Role of Electrons
In addition to lattice vibrations, the same delocalized electrons that carry electrical charge also carry and transfer thermal energy. This dual-purpose role further enhances graphite's ability to dissipate heat.
Understanding the Trade-offs: Anisotropic Conduction
A critical concept for any practical application is that graphite's conductivity is anisotropic, meaning it is not the same in all directions.
High Conduction Along the Layers
Electricity and heat travel with exceptional ease parallel to the graphene sheets. This is the path of least resistance, where the delocalized electrons and lattice vibrations can move freely.
Poor Conduction Between the Layers
In contrast, conduction perpendicular to the layers is significantly worse. Electrons and vibrations must "jump" across the weak van der Waals gaps between the sheets, a much less efficient process. This results in much higher resistance and lower thermal conductivity in this direction.
Making the Right Choice for Your Goal
Understanding graphite's directional conductivity is essential for using it effectively.
- If your primary focus is electrical applications (like electrodes or battery anodes): You must orient the material so that the electrical current flows along the graphite layers for maximum efficiency.
- If your primary focus is thermal management (like heat spreaders or sinks): The graphite must be positioned to conduct heat away from a source along its highly conductive planes.
- If your primary focus is high-temperature stability (like in furnaces): Its ability to withstand thermal shock is tied to its capacity to efficiently dissipate heat gradients along its layers, preventing stress build-up.
Graphite's unique structure makes it a remarkable material that uniquely bridges the gap between metals and non-metals.
Summary Table:
| Property | Conduction Mechanism | Key Characteristic |
|---|---|---|
| Electrical Conductivity | Delocalized electrons moving within 2D layers | Very low resistance along the planes |
| Thermal Conductivity | Lattice vibrations (phonons) & electron movement | Excellent heat dissipation along the planes |
| Anisotropic Nature | Direction-dependent conduction | High conductivity parallel to layers; Poor perpendicular to layers |
Leverage Graphite's Unique Properties in Your Lab
Understanding the anisotropic conductivity of graphite is crucial for maximizing performance in applications like furnace components, thermal management systems, and electrodes.
KINTEK specializes in high-performance lab equipment and consumables, including precision graphite products designed for optimal thermal and electrical performance. Our experts can help you select the right material and orientation for your specific laboratory needs, ensuring efficiency, durability, and reliability.
Ready to enhance your application with the right graphite solution? Contact our team today for a consultation and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
People Also Ask
- What is the basic principle of graphite furnace atomic absorption spectroscopy? Achieve Ultra-Trace Element Detection
- Why is graphite furnace more sensitive than flame? Unlocking Ultra-Trace Detection for Your Lab
- Does graphite conduct electricity when melted? Discover the Secrets of Liquid Carbon Conductivity
- What is the temperature of atomic absorption in graphite furnace? Mastering the Multi-Stage Heating Program
- What is special about graphite? Unlocking Its Unique Properties for Extreme Applications
- How is synthetic graphite manufactured? A Deep Dive into the High-Temperature Process
- What are the benefits of a graphite furnace? Achieve Rapid, Uniform High-Temperature Processing
- What are the industrial uses of graphite? Leverage Its Unique Properties for Demanding Applications



















