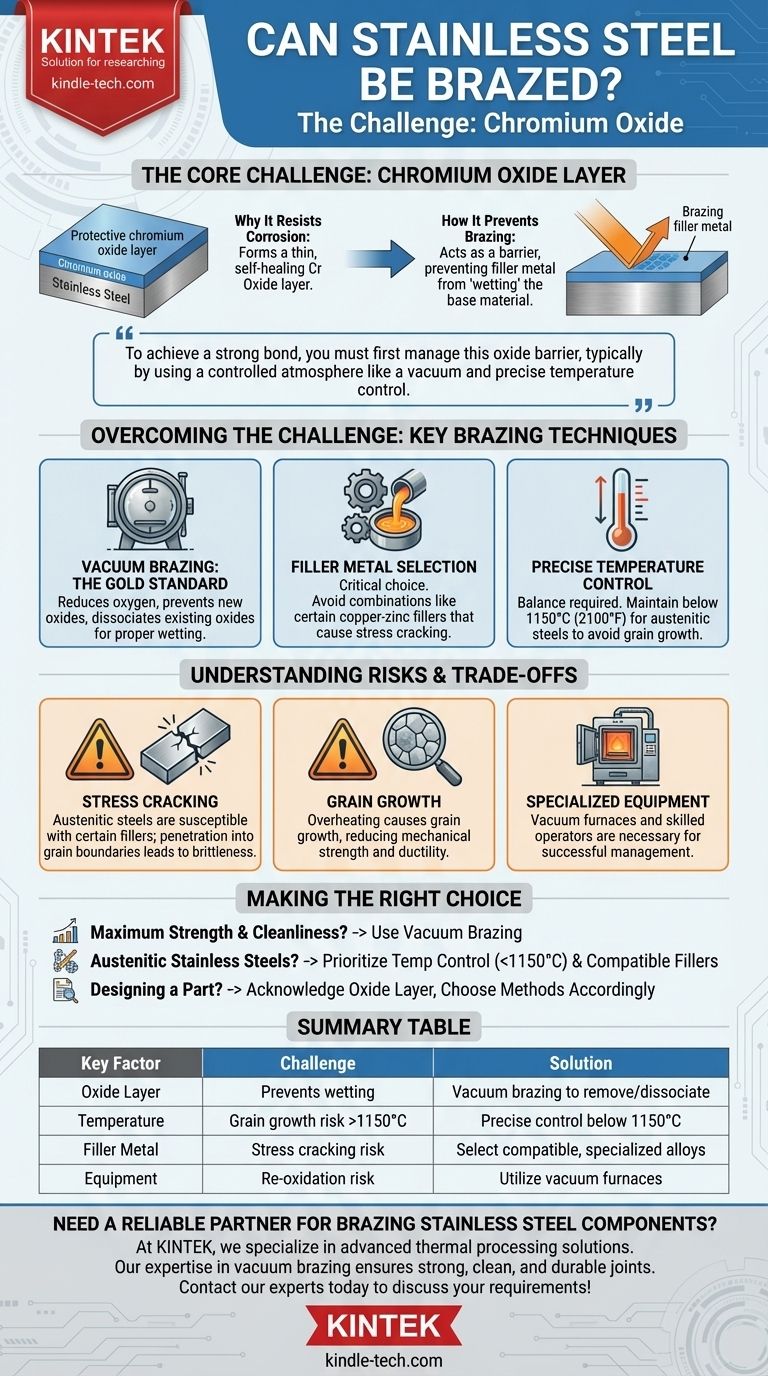
In short, yes, stainless steel can be brazed. However, the process is more complex than brazing simple carbon steels. Successfully brazing stainless steel hinges on overcoming the tenacious, invisible layer of chromium oxide that gives the material its signature corrosion resistance.
The core challenge of brazing stainless steel is not the steel itself, but its passive chromium oxide surface layer. To achieve a strong bond, you must first manage this oxide barrier, typically by using a controlled atmosphere like a vacuum and precise temperature control.

The Core Challenge: The Chromium Oxide Layer
The very property that makes stainless steel so valuable is also what makes it difficult to braze. Understanding this oxide layer is the key to a successful process.
Why Stainless Steel Resists Corrosion
Stainless steel contains a significant amount of chromium (Cr), often along with other elements like nickel (Ni) and molybdenum (Mo). The chromium reacts with oxygen in the air to form an extremely thin, stable, and self-healing layer of chromium oxide.
This passive layer protects the underlying steel from reacting with its environment, which is why it doesn't rust.
How the Oxide Layer Prevents Brazing
This protective oxide layer acts as a barrier that prevents brazing filler metals from "wetting" or flowing across the base material. A successful braze requires the filler metal to form a direct metallurgical bond with the parent steel, which is impossible if the oxide is in the way.
Overcoming the Challenge: Key Brazing Techniques
To achieve a sound braze, the chromium oxide must be removed before or during the heating process, and the part must be protected from re-oxidation.
Vacuum Brazing: The Gold Standard
Vacuum brazing is a highly effective method for joining stainless steel. By performing the process under a high vacuum, the amount of oxygen in the environment is drastically reduced.
This high-temperature, low-oxygen environment prevents the formation of new oxides. It can also cause existing oxides to dissociate or break down, allowing the filler metal to properly wet the clean steel surface. The result is a high-strength, clean, and aesthetically pleasing joint.
The Importance of Filler Metal Selection
The choice of filler metal is critical. Some combinations can create problems, even if the oxide layer is properly managed. For example, certain copper-zinc filler metals are known to cause stress cracking in austenitic stainless steels.
Precise Temperature Control
Temperature management is a delicate balance. While high temperatures are needed to break down oxides and melt the filler, excessive heat can damage the stainless steel itself. For austenitic stainless steels, temperatures should not exceed 1150°C (2100°F) to avoid excessive grain growth, which can weaken the material.
Understanding the Risks and Trade-offs
Brazing stainless steel is a precise technical process. Deviating from best practices introduces significant risks to the integrity of the final assembly.
Stress Cracking in Austenitic Steels
Austenitic stainless steels (like 304 or 316) are susceptible to intergranular stress cracking when brazed with certain filler metals. The filler metal can penetrate the grain boundaries of the steel, making it brittle and prone to failure under load.
The Risk of Grain Growth
Overheating stainless steel, even for a short time, can cause its internal crystalline structure (grains) to grow. Larger grains generally result in reduced mechanical strength and ductility, compromising the integrity of the part even if the brazed joint itself is strong.
The Need for Specialized Equipment
Successfully managing the oxide layer and controlling the temperature profile requires specialized equipment. Vacuum furnaces are a significant investment and require skilled operators, making this process less accessible than open-air torch brazing used for other metals.
Making the Right Choice for Your Application
Use these principles to guide your technical approach to brazing stainless steel.
- If your primary focus is maximum joint strength and cleanliness: Vacuum brazing is the superior method as it actively removes the oxide layer and prevents re-oxidation.
- If you are working with austenitic stainless steels: Prioritize careful temperature control below 1150°C and select filler metals specifically designed to be compatible, avoiding those known to cause stress cracking.
- If you are designing a part to be brazed: Acknowledge that the process is defined by overcoming the stable chromium oxide layer and choose your manufacturing methods accordingly.
By addressing the unique challenge of the chromium oxide layer, you can reliably create strong and durable brazed stainless steel joints.
Summary Table:
| Key Factor | Challenge | Solution |
|---|---|---|
| Oxide Layer | Chromium oxide prevents filler metal wetting | Use vacuum brazing to remove oxygen and dissociate oxides |
| Temperature | Risk of grain growth and weakening above 1150°C | Maintain precise temperature control below 1150°C |
| Filler Metal | Risk of stress cracking with incompatible alloys | Select specialized, compatible filler metals |
| Equipment | Requires controlled atmosphere to prevent re-oxidation | Utilize vacuum furnaces for optimal results |
Need a reliable partner for brazing stainless steel components? At KINTEK, we specialize in advanced thermal processing solutions for laboratory and industrial applications. Our expertise in vacuum brazing ensures strong, clean, and durable joints for your stainless steel assemblies while preventing oxide formation and material degradation. Let us help you achieve perfect results with our precision equipment and technical knowledge. Contact our experts today to discuss your specific brazing requirements!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- How is the greatest joint strength obtained in brazing? Master the 3 Keys to Superior Metallurgical Bonds
- What is the basic of brazing? A Guide to Strong, Low-Heat Metal Joining
- What is the difference between welding and vacuum brazing? Choose the Right Joining Method for Your Project
- What is a vacuum furnace used for? Unlock Purity in High-Temperature Processing
- What is vacuum brazing? The Ultimate Guide to High-Purity, Flux-Free Metal Joining



















