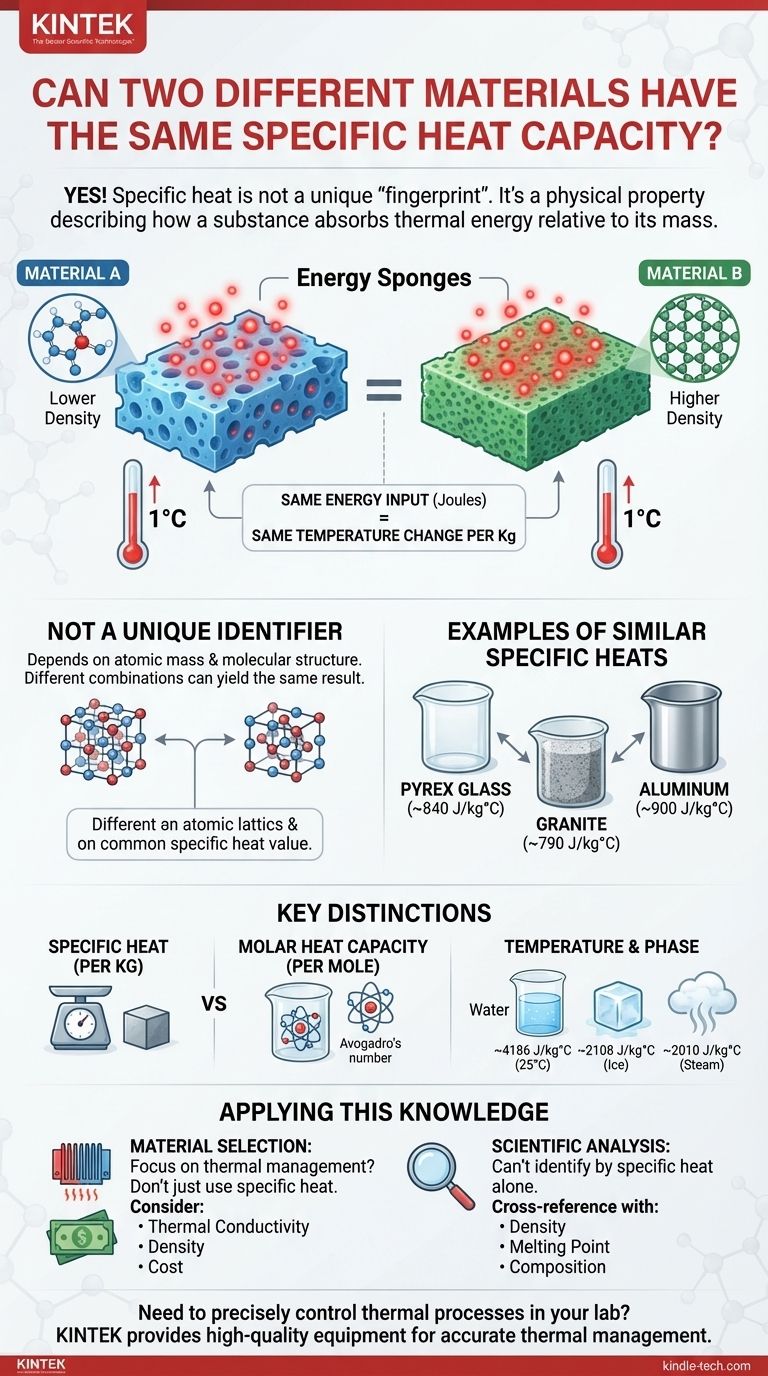
Yes, it is entirely possible for two different materials to have the same, or nearly identical, value of specific heat capacity. Specific heat capacity is not a unique "fingerprint" like an atomic number. Instead, it is a physical property that describes how a substance absorbs thermal energy relative to its mass, and different combinations of atomic mass and molecular structure can lead to the same result.
The core takeaway is that specific heat capacity is a measure of thermal behavior, not a fundamental identifier. It arises from complex interactions at the atomic level, and it is entirely possible for two distinct materials to exhibit the same behavior through different underlying structures.

What Specific Heat Capacity Truly Represents
An "Energy Sponge" Analogy
Think of specific heat capacity as the "absorbency" of a material's thermal sponge. It quantifies how much energy (in joules) you must add to one kilogram of a substance to raise its temperature by one degree Celsius (or Kelvin).
A material with a low specific heat, like copper (~385 J/kg°C), is like a small, dense sponge. It gets "saturated" with energy very quickly, meaning its temperature rises fast.
A material with a high specific heat, like water (~4186 J/kg°C), is like a massive, absorbent sponge. It can soak up a huge amount of energy before its temperature increases significantly.
Why It's Not a Unique Identifier
Specific heat is a macroscopic property that results from microscopic factors, primarily:
- Atomic Mass: Heavier atoms generally mean fewer atoms in a kilogram of material.
- Molecular Structure: The way atoms are bonded together determines how they can store energy through vibrations and rotations (their "degrees of freedom").
Because specific heat depends on the interplay of these factors, different combinations can coincidentally produce the same value. A material with lighter atoms but very strong bonds might end up having the same specific heat as one with heavier atoms and weaker bonds.
Examples of Similar Specific Heats
While finding two materials with the exact same value is rare, many have values that are extremely close and practically indistinguishable for engineering purposes.
Common Materials
Consider these values (at room temperature):
- Pyrex Glass: ~840 J/kg°C
- Granite: ~790 J/kg°C
- Aluminum: ~900 J/kg°C
Here, glass and granite have very similar capacities to absorb and store heat on a per-mass basis, despite being entirely different substances.
Advanced Materials
The principle is even clearer with engineered materials. It is possible to create alloys or composites with specific thermal properties. An engineer could intentionally design a material to match the specific heat of another substance for a particular application.
Key Distinctions and Considerations
Specific Heat vs. Molar Heat Capacity
This is the most critical distinction. While specific heat is measured per unit of mass (per kilogram), molar heat capacity is measured per unit of substance (per mole).
A mole is a fixed number of atoms or molecules (Avogadro's number). For many simple solid elements, the molar heat capacity is surprisingly similar (the Dulong-Petit law).
This tells us that on a per-atom basis, many materials absorb a similar amount of energy. The main reason their specific heats (per kilogram) are so different is that their atoms have different masses.
The Influence of Temperature and Phase
A material's specific heat is not a fixed constant. It changes with temperature and changes dramatically during phase transitions.
For example, the specific heat of water (~4186 J/kg°C) is nearly double that of ice (~2108 J/kg°C) or steam (~2010 J/kg°C). Therefore, two materials might share a specific heat value at 25°C but have very different values at 100°C.
How to Apply This Knowledge
For Material Selection
If your primary goal is thermal management (like in a heat sink or a thermal battery), you cannot choose a material based on specific heat alone.
A high specific heat is good for storing thermal energy, but you must also consider thermal conductivity (how fast it absorbs/releases energy), density (how much mass fits in a given volume), and cost. The fact that multiple materials can have similar specific heats gives you the flexibility to optimize for these other critical factors.
For Scientific Analysis
You can never definitively identify an unknown substance by measuring only its specific heat. It provides a clue but is not conclusive proof.
Proper identification requires cross-referencing multiple properties, such as density, melting point, thermal conductivity, and chemical composition.
Making the Right Interpretation
Understanding this concept allows you to use material properties more effectively.
- If your primary focus is engineering: Recognize that specific heat is a performance metric, and multiple materials may meet your thermal requirements, allowing you to optimize for other factors like weight, conductivity, or cost.
- If your primary focus is scientific analysis: Use specific heat as one property among many to characterize a substance, but never rely on it in isolation for identification.
Ultimately, viewing a material's properties as a description of its behavior, rather than a fixed identity, is the key to deeper understanding and innovation.
Summary Table:
| Material | Specific Heat Capacity (J/kg°C) | Key Insight |
|---|---|---|
| Pyrex Glass | ~840 | Similar values show specific heat is not a unique fingerprint. |
| Granite | ~790 | Different substances can have nearly identical thermal behavior. |
| Water | ~4186 | High specific heat is good for energy storage, but other factors matter. |
| Aluminum | ~900 | Material selection must also consider conductivity, density, and cost. |
Need to precisely control thermal processes in your lab?
Understanding material properties like specific heat capacity is crucial for reliable results. KINTEK specializes in providing high-quality lab equipment and consumables—from furnaces to thermal analysis tools—that help you accurately measure and manage heat transfer.
Let our expertise support your research and development. Contact our thermal specialists today to discuss your specific laboratory needs and find the right solution.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is ashing in a muffle furnace? Isolate Inorganic Content with Precision
- How does a muffle furnace work? A Guide to Clean, High-Temperature Heating
- What is a muffle furnace for laboratory use? A Guide to Contaminant-Free High-Temperature Processing
- How do you measure ash content? Choose the Right Method for Accurate Results
- What are the advantages and limitations of heat treatment? Tailor Material Properties for Peak Performance



















