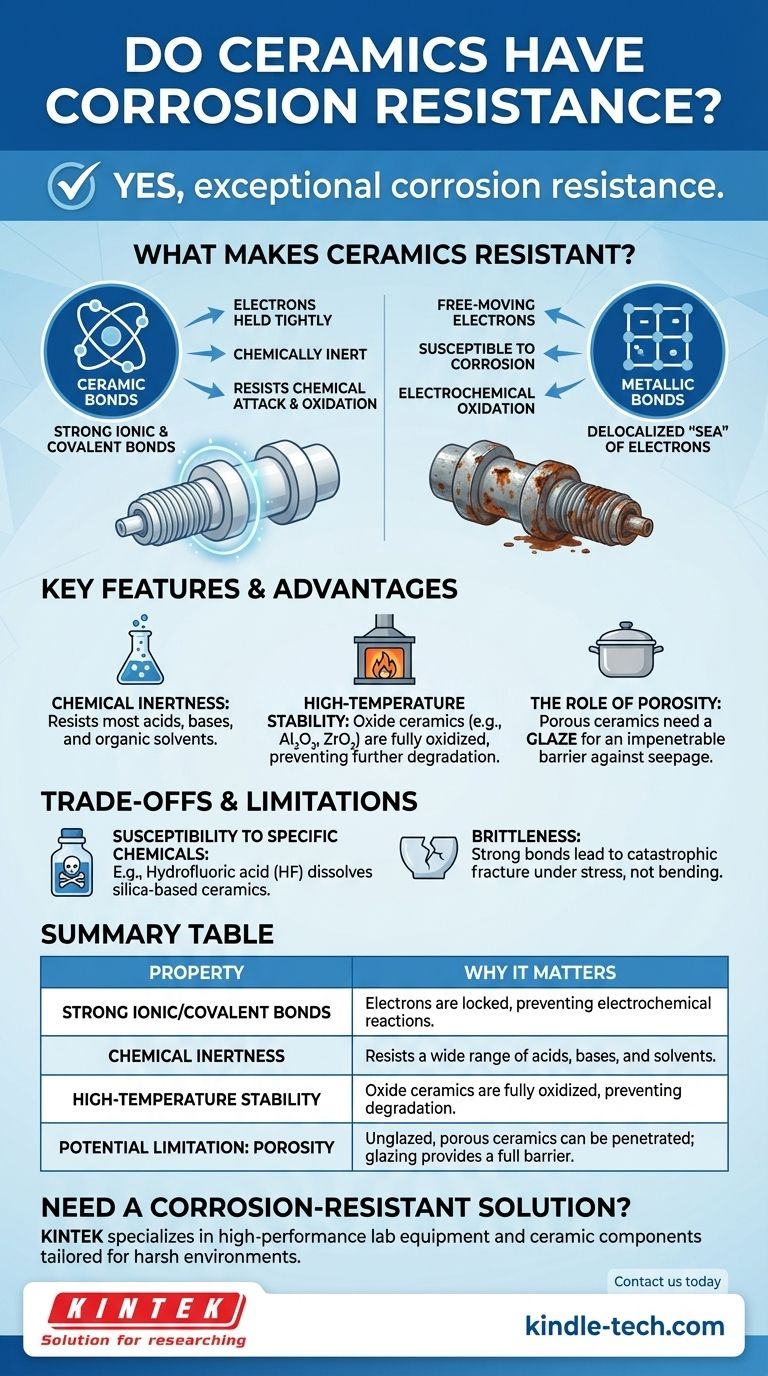
Yes, as a category, ceramics possess exceptional corrosion resistance. This chemical stability is one of their most defining characteristics and a primary reason for their use in demanding environments. Unlike metals, which corrode through electrochemical reactions, the strong chemical bonds in most ceramics make them inherently inert and resistant to chemical attack, oxidation, and high-temperature degradation.
The fundamental reason for a ceramic's corrosion resistance lies in its strong ionic and covalent chemical bonds. These bonds hold electrons tightly, preventing the chemical reactions—especially oxidation—that degrade other materials like metals.

What Makes Ceramics Corrosion Resistant?
To understand why ceramics are a go-to material for harsh environments, we need to look at their fundamental atomic structure and compare it to that of metals.
The Power of Chemical Bonds
Ceramics are characterized by very strong ionic and covalent bonds. These bonds involve either the transfer or sharing of electrons between atoms, resulting in a very stable, low-energy state. The electrons are held tightly in place and are not free to move.
In contrast, metals have metallic bonds, where electrons form a delocalized "sea" that moves freely around a lattice of positive ions. This mobility is what makes metals good conductors of electricity, but it also makes them highly susceptible to corrosion, which is an electrochemical process of losing electrons (oxidation).
Inherent Chemical Inertness
Because the electrons in a ceramic are locked so securely in their bonds, the material does not readily react with its environment. It is chemically inert. This makes most ceramics highly resistant to a wide range of corrosive agents, including most acids, bases, and organic solvents.
Stability at High Temperatures
Many of the most durable technical ceramics are oxides (like aluminum oxide or zirconium oxide). These materials are already in their highest-possible oxidized state. They cannot be oxidized further, which gives them phenomenal resistance to the high-temperature oxidation and scaling that destroys metals in environments like furnaces or engine components.
Understanding the Trade-offs and Limitations
While ceramics are exceptionally resistant, they are not universally immune to all forms of attack. Understanding their limitations is critical for proper material selection.
Susceptibility to Specific Chemicals
Certain highly aggressive chemicals can attack specific ceramics. For example, hydrofluoric acid is known to dissolve silica-based ceramics, and certain molten salts or metals at very high temperatures can also cause degradation. The key is matching the specific ceramic to the specific chemical environment.
The Role of Porosity
Traditional ceramics, like the terracotta used in cooking vessels, can be porous. While the ceramic material itself is resistant, corrosive agents can seep into these pores, leading to internal damage or contamination. This is why such products are often sealed with a glaze—a non-porous glassy layer that provides a truly impenetrable barrier.
Brittleness as a Design Constraint
The primary trade-off for the hardness and chemical stability of ceramics is brittleness. The same strong, rigid bonds that prevent corrosion also prevent plastic deformation. This means that under stress, ceramics tend to fracture catastrophically rather than bend. This is not a form of corrosion, but it is the most critical design constraint to consider when using them.
Making the Right Choice for Your Application
Selecting the correct material requires aligning the type of ceramic with the specific environmental challenge it will face.
- If your primary focus is resisting high-temperature oxidation: Choose oxide ceramics like alumina (Al₂O₃) or zirconia (ZrO₂), as they are already fully oxidized and exceptionally stable.
- If your primary focus is resisting aggressive chemical attack: You must match a specific technical ceramic, such as silicon carbide (SiC), to the specific chemical agent, as resistance can vary.
- If you are using traditional ceramics for general-purpose use: Ensure the product has a high-quality, non-porous glaze to provide a complete barrier against chemical absorption.
By understanding the unique properties of their chemical bonds, you can confidently leverage ceramics for performance in environments where most other materials would fail.
Summary Table:
| Property | Why It Matters for Corrosion Resistance |
|---|---|
| Strong Ionic/Covalent Bonds | Electrons are locked in place, preventing the electrochemical reactions that cause corrosion. |
| Chemical Inertness | Resists attack from a wide range of acids, bases, and solvents. |
| High-Temperature Stability | Oxide ceramics are already fully oxidized, preventing further degradation in extreme heat. |
| Potential Limitation: Porosity | Unglazed, porous ceramics can allow corrosive agents to seep in; glazing provides a full barrier. |
Need a corrosion-resistant solution for your lab? The exceptional chemical stability of ceramics makes them ideal for harsh environments, from handling aggressive chemicals to high-temperature processes. At KINTEK, we specialize in providing high-performance lab equipment and consumables, including ceramic components tailored to withstand your specific application challenges. Contact us today via our [#ContactForm] to discuss how our materials can enhance the durability and reliability of your laboratory operations.
Visual Guide
Related Products
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Hexagonal Boron Nitride HBN Ceramic Ring
People Also Ask
- Which is harder silicon carbide or tungsten carbide? Discover the Key to Material Selection
- What are the properties and applications of silicon carbide ceramics? Solve Extreme Engineering Challenges
- What is the temperature resistance of silicon carbide? Withstands Extreme Heat Up to 1500°C
- What is the resistivity of silicon carbide? It's a tunable property from <0.1 ohm-cm to highly resistive.
- What is the strongest ceramics? Silicon Carbide Leads in Hardness & Thermal Strength



















