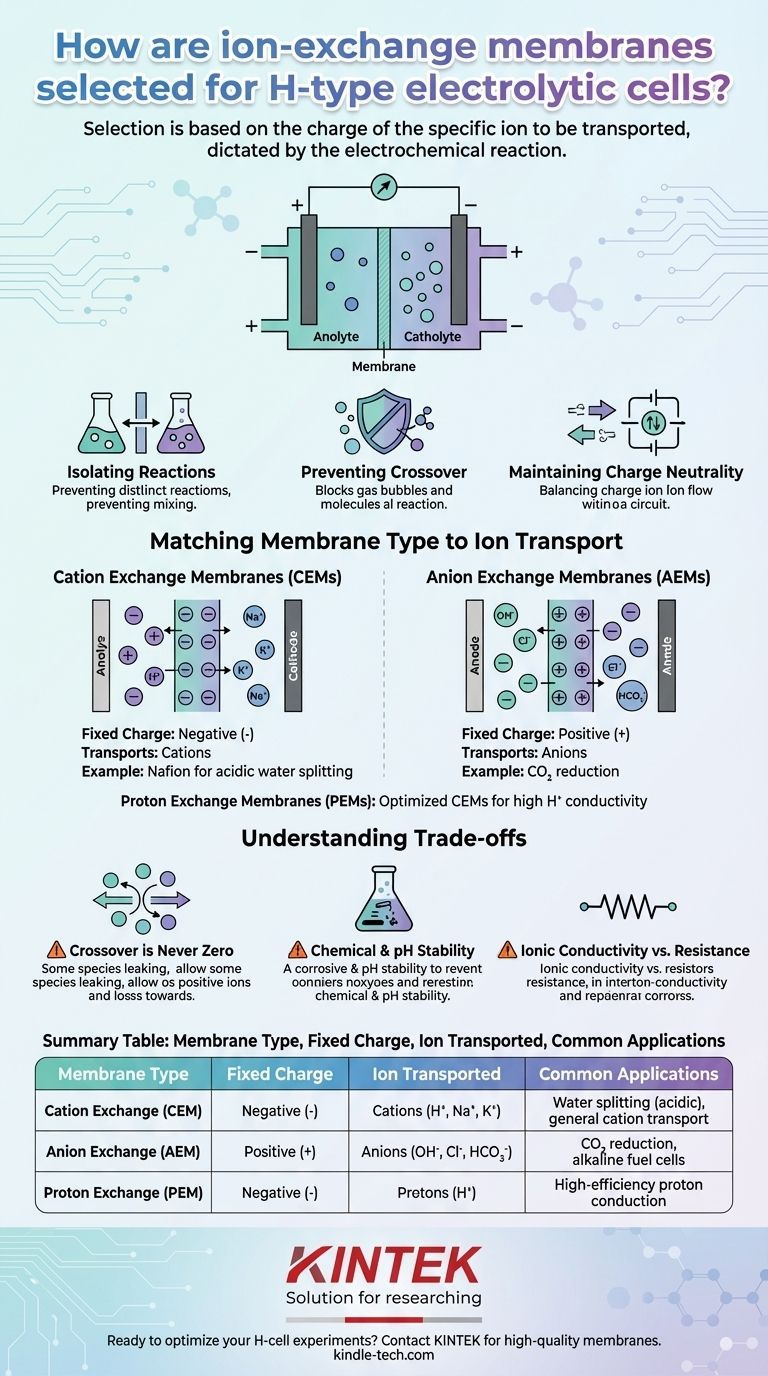
The right ion-exchange membrane is selected based on the charge of the specific ion you need to transport between the two chambers of your H-type cell. This choice is dictated by the electrochemical reaction you are studying. You must choose a membrane that selectively allows the passage of either positive ions (cations) or negative ions (anions) to balance the charge generated at the electrodes, while simultaneously preventing the unwanted mixing of reactants and products.
Choosing a membrane is not a passive component choice; it is a strategic decision that defines the electrochemical environment of your experiment. The membrane's primary function is to complete the electrical circuit by shuttling specific ions, thereby isolating the anode and cathode reactions to ensure the purity and efficiency of your target process.

The Fundamental Role of the Membrane in an H-Cell
An H-type cell is designed to physically separate the two electrode compartments (the anolyte and catholyte). The membrane is the critical barrier that connects them electrochemically.
Isolating Anode and Cathode Reactions
The membrane creates two distinct micro-environments. This allows you to study a specific reaction at one electrode without interference from the simultaneous reaction occurring at the other.
Preventing Product Crossover
Many electrochemical processes produce gases or soluble species. The membrane's job is to block these products from migrating to the other chamber where they could react, poison the catalyst, or complicate analysis.
Maintaining Charge Neutrality
As electrons flow through the external circuit, ions must flow through the electrolyte and across the membrane to prevent charge buildup. The membrane ensures this internal ionic current is carried by a specific type of ion, completing the circuit.
Matching Membrane Type to Ion Transport
The core of your decision lies in identifying which ion needs to move to balance the charge from your reaction.
Cation Exchange Membranes (CEMs)
These membranes contain fixed, negatively charged functional groups (like sulfonate, –SO₃⁻) within their polymer structure.
This static negative charge repels anions but allows positive ions (cations) like H⁺, K⁺, or Na⁺ to pass through, moving toward the negatively charged cathode.
A classic example is Nafion, which is highly selective for proton (H⁺) transport and is the standard for water electrolysis in acidic conditions.
Anion Exchange Membranes (AEMs)
Conversely, AEMs contain fixed, positively charged functional groups (like quaternary ammonium, –NR₃⁺).
These fixed positive charges repel cations but allow negative ions (anions) like OH⁻, Cl⁻, or HCO₃⁻ to pass through, moving toward the positively charged anode.
AEMs are often used in CO₂ reduction experiments where transporting anions like bicarbonate can help maintain a favorable pH near the cathode.
Proton Exchange Membranes (PEMs)
This term is often used interchangeably with CEMs but specifically refers to membranes optimized for high proton (H⁺) conductivity. While all PEMs are a type of CEM, not all CEMs are efficient PEMs.
Understanding the Trade-offs and Key Pitfalls
Selecting a membrane involves more than just matching the ion charge. You must consider the practical limitations that can impact your results.
Crossover is Never Zero
No membrane is a perfect barrier. Small amounts of neutral molecules (like dissolved O₂, CO₂, or methanol) and even some non-target ions can slowly diffuse across, a phenomenon known as crossover.
This can lead to side reactions or lower the measured efficiency (Faradaic efficiency) of your primary reaction.
Chemical and pH Stability
The membrane must be chemically stable in your chosen electrolyte and at the potentials you apply.
AEMs, for example, can be susceptible to degradation in highly alkaline (high pH) environments, while the oxidative environment at the anode can be harsh on many polymer backbones.
Ionic Conductivity vs. Resistance
A membrane's effectiveness is also measured by its ionic conductivity—how easily the target ion can pass through.
Low conductivity means high ionic resistance, which adds to the overall voltage required to drive your reaction, representing a loss in energy efficiency.
Making the Right Choice for Your Goal
Your experimental goal is the ultimate guide for membrane selection.
- If your primary focus is water splitting in acidic media: A cation exchange membrane (specifically a PEM like Nafion) is the standard choice to efficiently transport protons (H⁺) from the anode to the cathode.
- If your primary focus is CO₂ reduction in a neutral electrolyte: An anion exchange membrane is often preferred to transport anions (e.g., HCO₃⁻) and help buffer the local pH at the cathode, suppressing the competing hydrogen evolution reaction.
- If your primary focus is separating two distinct redox couples: Choose a membrane that allows the supporting electrolyte's ion to pass (e.g., K⁺ through a CEM) while blocking the larger, active redox species in each half-cell.
Ultimately, the correct membrane enables clean, well-defined electrochemistry by controlling the very medium in which the reaction occurs.
Summary Table:
| Membrane Type | Fixed Charge | Ion Transported | Common Applications |
|---|---|---|---|
| Cation Exchange (CEM) | Negative (-) | Cations (H⁺, Na⁺, K⁺) | Water splitting (acidic), general cation transport |
| Anion Exchange (AEM) | Positive (+) | Anions (OH⁻, Cl⁻, HCO₃⁻) | CO₂ reduction, alkaline fuel cells |
| Proton Exchange (PEM) | Negative (-) | Protons (H⁺) | High-efficiency proton conduction (e.g., Nafion) |
Ready to optimize your H-cell experiments with the perfect ion-exchange membrane? KINTEK specializes in high-quality lab equipment and consumables, including membranes tailored for electrochemistry research. Our experts can help you select the right membrane to ensure high Faradaic efficiency, minimal crossover, and stable performance. Contact us today to discuss your specific application and elevate your laboratory's capabilities!
Visual Guide
Related Products
- Anion Exchange Membrane for Laboratory Use
- Proton Exchange Membrane for Batteries Lab Applications
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Polyethylene Separator for Lithium Battery
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
People Also Ask
- What types of ion-exchange membranes can be used with the H-type electrolytic cell? Select the Best Ion Barrier
- What affects melting point chemistry? A Guide to Molecular Forces and Lattice Energy
- What is the primary function of a porous diaphragm in AWE? Key Roles in Gas Separation & Ion Flow
- What is the purpose of an anion exchange membrane (AEM) or PEM? Enhance Electrochemical Efficiency
- How do ion-exchange membranes prevent H2O2 decomposition? Boost Yield and Efficiency in Flow Cells














