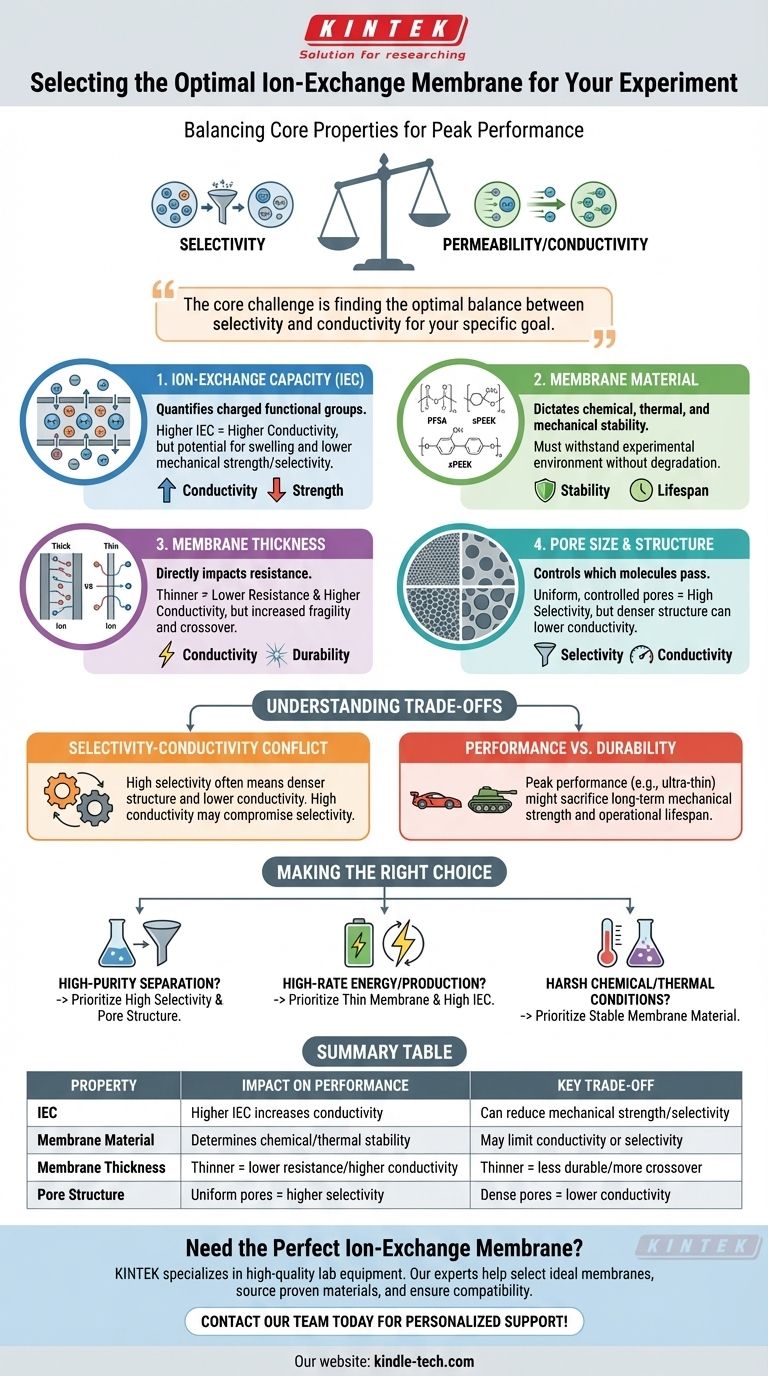
To select the right ion-exchange membrane, you must evaluate four primary physical characteristics: the membrane's material, its ion-exchange capacity (IEC), its thickness, and its pore structure. These factors collectively determine the two most critical performance metrics for any experiment: how well the membrane selects for the desired ions (selectivity) and how easily those ions can pass through it (permeability or conductivity).
The core challenge in selecting an ion-exchange membrane is not finding the "best" one, but rather finding the optimal balance between conflicting properties—primarily selectivity and conductivity—that best serves the specific goal of your experiment.

The Two Pillars of Membrane Performance
Every physical characteristic of a membrane is chosen to optimize two fundamental, often competing, performance outcomes. Understanding these goals is the first step in making an informed choice.
What is Selectivity?
Selectivity is the membrane's ability to discriminate between ions, allowing certain ions to pass through while blocking others.
High selectivity is critical in applications like desalination, where you need to separate salt ions from water, or in electrodialysis for purifying specific chemicals.
What is Permeability (and Conductivity)?
Permeability, or its electrical equivalent, ionic conductivity, measures how easily and quickly ions can travel through the membrane.
High conductivity is essential for processes where efficiency and high throughput are key, such as in fuel cells or chlor-alkali production, as it directly relates to lowering the electrical resistance of the system.
Core Physical Properties and Their Impact
The physical makeup of the membrane directly controls its performance. Here is how the key properties you must consider influence the outcome of your experiment.
Ion-Exchange Capacity (IEC)
IEC quantifies the number of charged functional groups within the membrane material. It is a measure of the membrane's theoretical charge-carrying ability.
A higher IEC generally leads to higher ionic conductivity because there are more sites to facilitate ion transport. However, it can also cause the membrane to swell more in water, potentially reducing its mechanical strength and selectivity.
Membrane Material
The base polymer of the membrane dictates its fundamental chemical, thermal, and mechanical stability. Common materials include perfluorosulfonic acid (PFSA) polymers like Nafion or sulfonated polyether ether ketone (sPEEK).
The choice of material is your first filter. You must select a polymer that can withstand the chemical environment and temperature of your experiment without degrading.
Membrane Thickness
Thickness has a direct and significant impact on resistance. A thinner membrane will have a shorter path for ions to travel, resulting in lower resistance and higher conductivity.
However, thinner membranes are often more fragile and can be more susceptible to crossover, where unwanted molecules or ions leak through.
Pore Size and Structure
The microscopic channels within the membrane control which molecules can pass through. The size, shape, and distribution of these pores are critical.
Tightly controlled, uniform pores are essential for high selectivity, ensuring that only ions below a certain size can pass. Inconsistent or large pores can lead to poor separation performance.
Understanding the Trade-offs
There is no universally perfect membrane. Your selection will always involve balancing competing characteristics to fit your specific application.
The Selectivity-Conductivity Conflict
This is the most common trade-off you will face. A membrane designed for extremely high selectivity often has a denser structure or lower IEC, which increases its resistance and lowers its conductivity.
Conversely, a membrane optimized for high conductivity may have a more open structure or higher water uptake, which can compromise its ability to perfectly select between similar ions.
Performance vs. Durability
Another key trade-off is between peak performance and operational lifespan. An ultra-thin membrane might offer exceptional conductivity, but it may not have the mechanical strength to survive long-term operation or pressure differentials.
Thicker, more robust membranes provide stability and a longer lifespan at the cost of higher electrical resistance and potentially lower efficiency.
Making the Right Choice for Your Experiment
Your experimental goal must be the deciding factor. Use your primary objective to prioritize which membrane properties are non-negotiable and which can be compromised.
- If your primary focus is high-purity separation: Prioritize a membrane with high selectivity and a well-defined pore structure, even if it means accepting lower conductivity.
- If your primary focus is high-rate energy or production processes: Prioritize a thin membrane with high ion-exchange capacity to maximize conductivity and minimize energy loss.
- If your primary focus is operating in harsh chemical or thermal conditions: Prioritize the membrane's base material for its stability, as this will determine the fundamental viability of the experiment.
Ultimately, a successful experiment depends on choosing a membrane whose properties are precisely aligned with your intended outcome.
Summary Table:
| Property | Impact on Performance | Key Trade-off |
|---|---|---|
| Ion-Exchange Capacity (IEC) | Higher IEC increases conductivity | Can reduce mechanical strength/selectivity |
| Membrane Material | Determines chemical/thermal stability | May limit conductivity or selectivity |
| Membrane Thickness | Thinner = lower resistance/higher conductivity | Thinner = less durable/more crossover |
| Pore Structure | Uniform pores = higher selectivity | Dense pores = lower conductivity |
Need the Perfect Ion-Exchange Membrane for Your Experiment?
Choosing the right membrane is critical for achieving accurate results in applications like electrodialysis, fuel cells, or chemical purification. At KINTEK, we specialize in providing high-quality lab equipment and consumables, including ion-exchange membranes tailored to your specific research needs.
Our experts can help you:
- Select membranes with the ideal balance of selectivity and conductivity
- Source materials proven for chemical and thermal stability
- Ensure compatibility with your experimental conditions
Let us help you optimize your lab's performance. Contact our team today for personalized recommendations and support!
Visual Guide
Related Products
- Anion Exchange Membrane for Laboratory Use
- Proton Exchange Membrane for Batteries Lab Applications
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What types of ion-exchange membranes can be used with the H-type electrolytic cell? Select the Best Ion Barrier
- How do ion-exchange membranes prevent H2O2 decomposition? Boost Yield and Efficiency in Flow Cells
- What affects melting point chemistry? A Guide to Molecular Forces and Lattice Energy
- What is the primary function of a Cation Exchange Membrane? Optimize Cu-Cl Cycle Efficiency and Longevity
- What is the purpose of an anion exchange membrane (AEM) or PEM? Enhance Electrochemical Efficiency



















