Before first use, a new proton exchange membrane (PEM) requires three essential steps: careful physical inspection for defects, removal of its protective films, and chemical pre-treatment to ensure it is clean and fully activated for optimal proton conductivity. These initial procedures are not optional; they are critical for the performance and longevity of the membrane in any electrochemical application.
The preparation of a new proton exchange membrane is not merely about unwrapping it. It is a systematic process of physical verification and chemical purification designed to transform the membrane from an inert, as-shipped component into a functionally reliable and high-performance heart of your electrochemical cell.

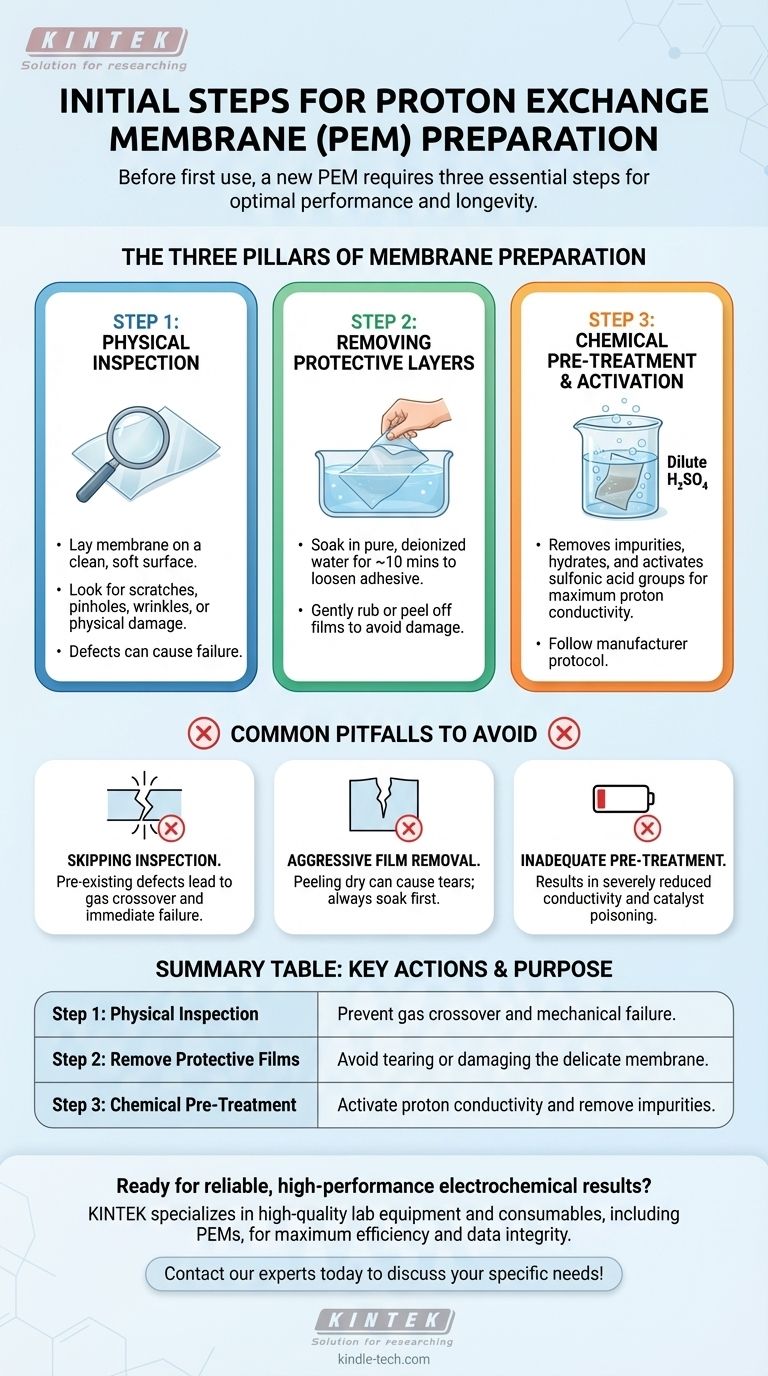
The Three Pillars of Membrane Preparation
Properly preparing a new membrane is foundational. Skipping any of these steps introduces significant risk of immediate failure, underperformance, or unreliable data.
Step 1: Physical Inspection
Before you do anything else, you must carefully inspect the membrane. It is a delicate component, and defects introduced during manufacturing or shipping can render it useless.
Lay the membrane on a clean, soft surface. Look for any scratches, pinholes, wrinkles, or physical damage. A defect can create a path for fuel crossover or a point of mechanical failure under operating pressure. If any significant issues are found, the membrane should be replaced.
Step 2: Removing Protective Layers
Most new membranes are shipped with protective plastic films on both sides. These must be removed without damaging the membrane itself.
The safest method is to soak the membrane in pure, deionized water for approximately ten minutes. This allows the membrane to swell slightly, which helps loosen the adhesive on the protective films. After soaking, you should be able to gently rub or peel the films off.
Step 3: Chemical Pre-Treatment and Activation
This is the most critical step for performance. Pre-treatment serves two primary goals: removing organic or metallic impurities from the manufacturing process and fully hydrating and activating the sulfonic acid groups responsible for proton transport.
A common procedure involves soaking the membrane in a solution like dilute sulfuric acid. This process ensures the membrane is in a fully protonated form and maximizes its ionic conductivity. Always follow the specific pre-treatment protocol recommended by the membrane manufacturer.
Common Pitfalls in Membrane Preparation
Mistakes made during these initial steps are a frequent source of poor fuel cell or electrolyzer performance. Understanding them is key to avoiding wasted time and resources.
Skipping the Inspection
Installing a membrane with a pre-existing defect, like a small scratch, almost guarantees failure. It can lead to high gas crossover, creating a safety hazard and rendering experimental results invalid.
Aggressive Film Removal
Attempting to peel the protective films off a dry membrane can easily cause it to tear or stretch. The water-soaking step is not just a suggestion; it is a necessary precaution to prevent mechanical damage.
Inadequate Pre-Treatment
Using a membrane without proper chemical activation will result in severely reduced proton conductivity. Impurities left on the membrane can also poison the catalyst layers in the electrode assembly, permanently degrading the performance of the entire device.
Making the Right Choice for Your Goal
The rigor of your preparation should match the goal of your work.
- If your primary focus is maximum performance and data reliability: A complete, multi-step chemical pre-treatment protocol (e.g., acid and water boiling) is non-negotiable.
- If your primary focus is rapid screening or educational demonstration: Simple hydration in deionized water after inspection may be sufficient, but you must accept that the performance will be suboptimal.
Ultimately, proper preparation is the foundation upon which all reliable electrochemical measurements are built.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Physical Inspection | Check for scratches, pinholes, or wrinkles. | Prevent gas crossover and mechanical failure. |
| 2. Remove Protective Films | Soak in deionized water, then gently peel. | Avoid tearing or damaging the delicate membrane. |
| 3. Chemical Pre-Treatment | Soak in dilute sulfuric acid (or as specified). | Activate proton conductivity and remove impurities. |
Ready to achieve reliable, high-performance results with your electrochemical equipment?
Proper membrane preparation is just the beginning. KINTEK specializes in providing the high-quality lab equipment and consumables—including PEMs and related electrochemical cell components—that your research demands. Our expertise ensures you have the right tools and support for maximum efficiency and data integrity.
Let's optimize your lab's capabilities. Contact our experts today to discuss your specific needs!
Visual Guide
Related Products
- Proton Exchange Membrane for Batteries Lab Applications
- Anion Exchange Membrane for Laboratory Use
- Polyethylene Separator for Lithium Battery
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Thermally Evaporated Tungsten Wire for High Temperature Applications
People Also Ask
- What operating conditions must be controlled when using a proton exchange membrane? Master Temperature, Humidity, and Pressure
- What is the function of perfluorinated sulfonic acid proton exchange membranes in the preparation of biomimetic sensors?
- What are the procedures for handling a proton exchange membrane after use? Ensure Longevity and Performance
- How should a proton exchange membrane be installed? A Guide to Flawless Assembly for Peak Performance
- Why is humidity control critical for PEM maintenance? Achieve Peak Performance and Longevity
















