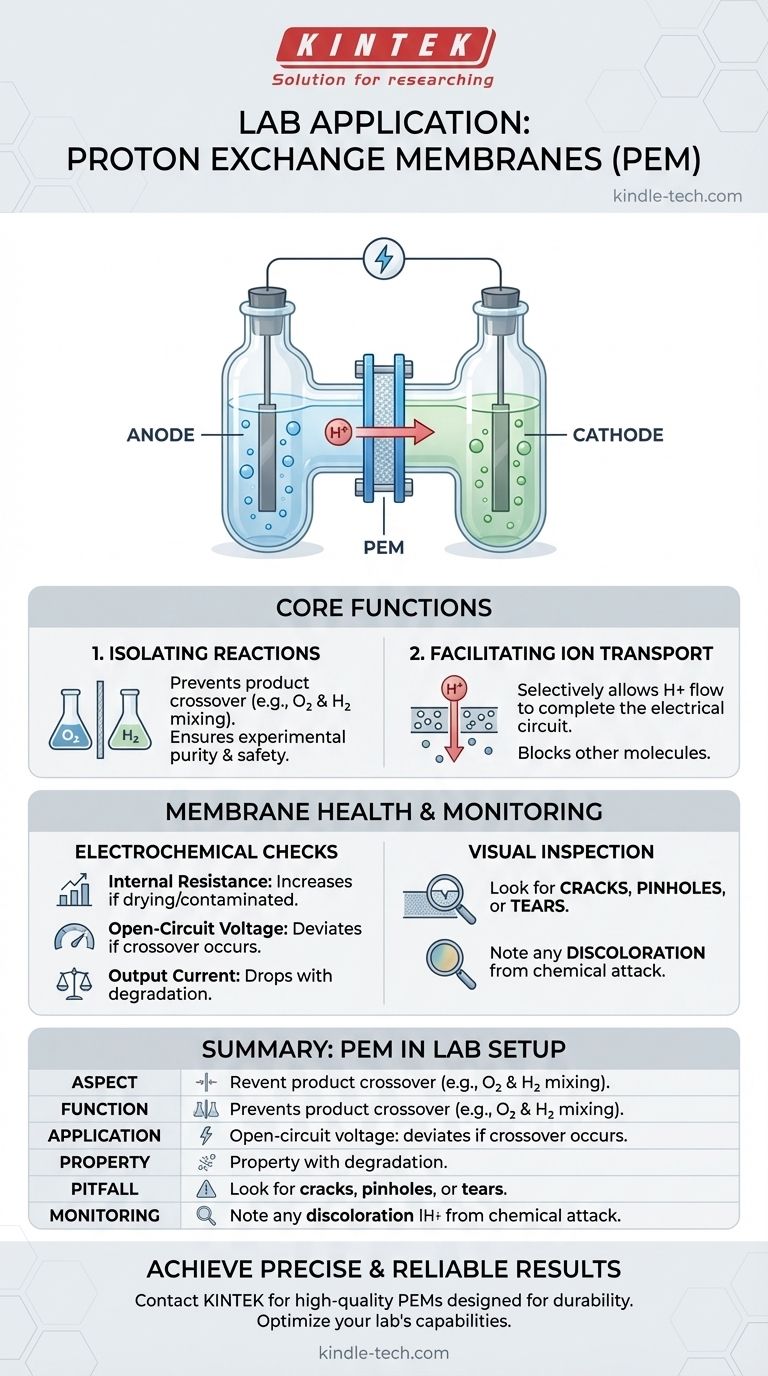
In laboratory environments, the most common application for a proton exchange membrane (PEM) is to serve as a selective barrier separating the anode and cathode chambers within an electrochemical cell. This setup is most frequently seen in H-type or triple-H-type electrolytic cells, where precise control over the two half-reactions is essential for research.
The core function of a PEM in a lab is not just to be a physical divider, but to act as a functional separator. It isolates the chemical environments of the anode and cathode to ensure experimental purity while selectively transporting protons to complete the electrical circuit.

The Role of the PEM in Electrolytic Cells
To understand the application, we must first understand the context of the hardware it's used in and the fundamental problem it solves.
What is an H-Type Cell?
An H-type electrolytic cell is a common piece of laboratory glassware named for its characteristic 'H' shape. It consists of two vertical chambers (one for the anode, one for the cathode) connected by a horizontal bridge.
The proton exchange membrane is clamped or fitted into this central bridge, effectively dividing the cell into two distinct compartments.
The Primary Function: Isolating Reactions
The main reason for separating the chambers is to prevent product crossover. In many electrochemical reactions, the products generated at the anode should not mix with the products at the cathode.
For example, in water splitting, oxygen is produced at the anode and hydrogen at the cathode. If these gases were allowed to mix, it would create an explosive mixture and make it impossible to measure the production of each gas accurately.
By isolating the chambers, the PEM ensures the purity of the products and prevents unwanted side reactions, allowing for precise and safe experimentation.
The Secondary Function: Facilitating Ion Transport
While it acts as a physical barrier to molecules and gases, the PEM is specifically designed to be permeable to certain ions—in this case, protons (H+).
This selective transport is critical. As the electrochemical reaction proceeds, an electrical circuit must be completed. The PEM allows protons to travel from the anode chamber to the cathode chamber, balancing the charge and allowing the reaction to continue.
Common Pitfalls and Membrane Health
A compromised membrane can invalidate experimental results. Therefore, knowing how to monitor its condition is a critical part of its application in the lab.
Why Monitoring is Crucial
A crack, tear, or degradation of the membrane can lead to the mixing of reactants and products, defeating its primary purpose. A change in its chemical structure can impede proton flow, increasing resistance and altering the experiment's energetics.
Electrochemical Health Checks
You can monitor the membrane's performance by periodically checking the cell's key parameters.
- Internal Resistance: A significant increase in resistance often indicates the membrane is drying out or has been contaminated.
- Open-Circuit Voltage: A deviation from the expected voltage can signal that unwanted crossover is occurring, creating a mixed potential.
- Output Current: A drop in current under a fixed voltage can point to increased resistance or other degradation issues within the membrane.
Visual Inspection
Before and after an experiment, a simple visual check is essential. Look for any physical damage, such as cracks, pinholes, or tears.
Also, note any discoloration. This can be a sign of chemical attack or contamination from metallic ions, which can permanently degrade the membrane's performance.
Ensuring a Successful Experiment
Applying this knowledge correctly depends on your experimental goals.
- If your primary focus is product purity: Ensure the membrane is properly sealed in the H-cell and is of a high grade to minimize any crossover of reactants or products.
- If your primary focus is long-term stability: Implement a routine of periodic electrochemical checks to track the membrane's health and replace it before degradation significantly impacts your results.
Ultimately, the proton exchange membrane is the component that enables precise and controlled electrochemical analysis in a laboratory setting.
Summary Table:
| Aspect | Role of the PEM in a Lab Setup |
|---|---|
| Primary Function | Isolates anode and cathode chambers to prevent product crossover and ensure experimental purity. |
| Key Application | Used in H-type or triple-H-type electrolytic cells for controlled electrochemical research. |
| Critical Property | Selectively transports protons (H+) to complete the electrical circuit while blocking other molecules. |
| Common Pitfall | Membrane degradation (cracks, contamination) can lead to mixed reactions and invalidate results. |
| Health Monitoring | Check internal resistance, open-circuit voltage, and perform visual inspections for damage or discoloration. |
Ready to achieve precise and reliable results in your electrochemical research?
The correct proton exchange membrane is critical for isolating reactions and ensuring product purity in your H-cell experiments. KINTEK specializes in high-quality lab equipment and consumables, including PEMs designed for durability and performance in demanding laboratory environments.
Let our experts help you select the perfect components for your setup. Contact KINTEK today to discuss your specific application needs and enhance your lab's capabilities!
Visual Guide
Related Products
- Proton Exchange Membrane for Batteries Lab Applications
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Customizable Fuel Cell Stack Components for Diverse Applications
People Also Ask
- What are the procedures for handling a proton exchange membrane after use? Ensure Longevity and Performance
- What is a proton exchange membrane? The Selective Heart of Hydrogen Energy Systems
- What operating conditions must be controlled when using a proton exchange membrane? Master Temperature, Humidity, and Pressure
- What should be done if a proton exchange membrane is found to be contaminated or damaged? Restore Performance or Replace for Safety
- What initial steps are required before using a new proton exchange membrane? Ensure Peak Performance and Longevity



















