In practice, a reducing atmosphere is created by introducing specific gases into a furnace that actively seek out and combine with free oxygen, thereby preventing the workpiece from oxidizing. The most common methods involve using the controlled combustion of fuel to generate a high CO-to-CO2 ratio, introducing a blend of pure gases like hydrogen and nitrogen, using dissociated ammonia as a source of hydrogen, or creating a high vacuum to remove oxygen-containing gases entirely.
The core challenge is not simply adding a "reducing gas," but maintaining a precise chemical imbalance where oxygen is actively removed faster than it can be introduced. This requires rigorous control over both the gas composition and the physical integrity of the furnace itself.

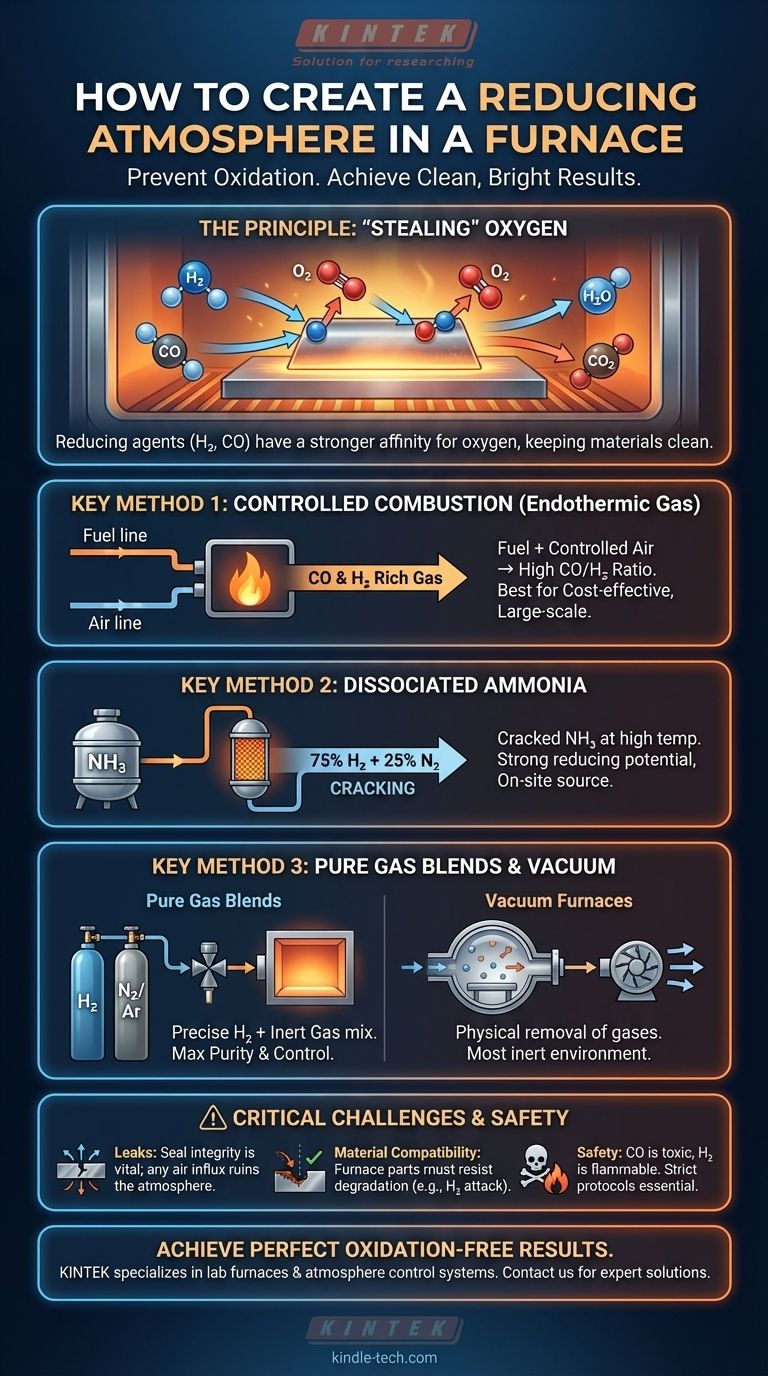
The Principle of a Reducing Atmosphere
A reducing atmosphere is a controlled furnace environment that is chemically engineered to prevent or reverse oxidation on the surface of a material during high-temperature processing.
Why It's Necessary
At elevated temperatures, most metals will readily react with any available oxygen to form oxides, which appear as scale or discoloration. A reducing atmosphere counteracts this by providing an abundance of "reducing agents."
How Reducing Agents Work
Reducing agents are elements or compounds, like hydrogen (H₂) and carbon monoxide (CO), that have a stronger affinity for oxygen than the material being treated. They effectively "steal" oxygen atoms from the environment and even from existing oxides on the material's surface, leaving it clean and bright.
Key Methods for Creating a Reducing Atmosphere
The specific method chosen depends on the material, the required purity, the process temperature, and cost considerations.
Method 1: Controlled Combustion (Endothermic Gas)
This is a widely used industrial method where a hydrocarbon fuel (like natural gas) is partially combusted with a controlled amount of air.
This reaction is managed to produce a gas rich in carbon monoxide (CO) and hydrogen (H₂), both powerful reducing agents. The ratio of CO to carbon dioxide (CO₂) is the critical control parameter.
Method 2: Dissociated Ammonia
In this process, anhydrous ammonia (NH₃) is cracked at high temperatures over a catalyst.
It decomposes into a mixture of 75% hydrogen and 25% nitrogen. The high concentration of hydrogen creates a very strong reducing potential.
Method 3: Pure Gas Blends
For maximum precision and purity, many processes use direct blends of pure bottled gases.
Commonly, this is a mixture of hydrogen (H₂) and an inert carrier gas like nitrogen (N₂) or argon (Ar). The percentage of hydrogen can be precisely controlled, from just a few percent for light reduction to 100% for aggressive applications.
Method 4: Vacuum Furnaces
A vacuum furnace creates a reducing environment by a different principle: physical removal of gases.
By pumping the furnace chamber down to a high vacuum, molecules—including oxygen—are almost entirely eliminated. This creates an inert environment that prevents oxidation, achieving a similar end result without a chemical reducing agent.
Understanding the Practical Challenges
Creating and maintaining the ideal atmosphere is a significant operational challenge that requires constant vigilance.
The Critical Threat of Leaks
A reducing atmosphere is only effective in a perfectly sealed furnace. Any leak, no matter how small, will allow air (which is 21% oxygen) to infiltrate the chamber.
This influx of oxygen will immediately compromise the atmosphere, potentially ruining the entire process. Regular leak detection and preventative maintenance are non-negotiable for reliable operation.
Material Compatibility at High Temperatures
The reducing gases themselves, combined with extreme heat, can be aggressive towards furnace components.
Materials like hydrogen can degrade certain metals and insulation. Furnace linings, tubes, and crucibles must be made of highly stable refractory materials like well-sintered alumina or magnesia to withstand the harsh chemical environment without softening or degrading.
Safety and Gas Handling
Many reducing agents are hazardous. Carbon monoxide is toxic, and hydrogen is highly flammable and explosive when mixed with air.
Proper safety protocols, ventilation, gas detection systems, and operator training are absolutely essential when working with these atmospheres.
Making the Right Choice for Your Process
Your choice of atmosphere depends directly on your technical requirements and operational constraints.
- If your primary focus is cost-effective, large-scale heat treatment: Endothermic gas from controlled combustion is a proven and economical industry standard.
- If your primary focus is high-purity processing with precise control: Blends of pure hydrogen and nitrogen offer the highest degree of chemical management.
- If your primary focus is preventing any surface reaction whatsoever: A high-vacuum furnace provides the most inert environment possible, free from chemical interaction.
- If your primary focus is a strong reducing potential from an on-site source: Dissociated ammonia provides a cost-effective supply of hydrogen-rich gas.
Ultimately, mastering your reducing atmosphere is a matter of precise chemical control and uncompromising equipment integrity.
Summary Table:
| Method | Key Components | Primary Use Case |
|---|---|---|
| Controlled Combustion | CO, H₂ from fuel/air mix | Cost-effective, large-scale heat treatment |
| Dissociated Ammonia | 75% H₂, 25% N₂ | Strong reduction from an on-site source |
| Pure Gas Blends | H₂, N₂, or Ar | High-purity processing with precise control |
| Vacuum Furnace | High Vacuum | Preventing any surface reaction |
Achieve perfect oxidation-free results in your lab. Creating and maintaining a precise reducing atmosphere is critical for successful heat treatment and sintering processes. KINTEK specializes in lab furnaces, atmosphere control systems, and the consumables needed for reliable operation. Our experts can help you select the right equipment and gases for your specific materials and application. Contact us today to discuss your requirements and ensure your furnace integrity and process control are uncompromising.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Why is an argon atmosphere furnace necessary for the long-term tempering of 12%Cr steel? Ensure Material Integrity
- How does a three-zone split atmosphere furnace ensure accuracy? Mastering Thermal Uniformity for Tensile Testing
- What is the function of an atmosphere protection annealing furnace? Optimize CoFe2O4/Fe Magnetic Performance
- Why is a precisely controlled high-temperature furnace with steam or air atmospheres required? Engineering Alpha-Alumina
- What is a sealed quench furnace? Achieve Precise, Clean Heat Treatment for Your Components
- What is a reducing atmosphere? Key Applications and Benefits for Industrial Processes
- What is furnace retort? Unlock Precise Heat Treatment with Controlled Atmospheres
- What is the primary function of an industrial atmosphere sintering furnace? Achieve Dense, High-Strength Components



















