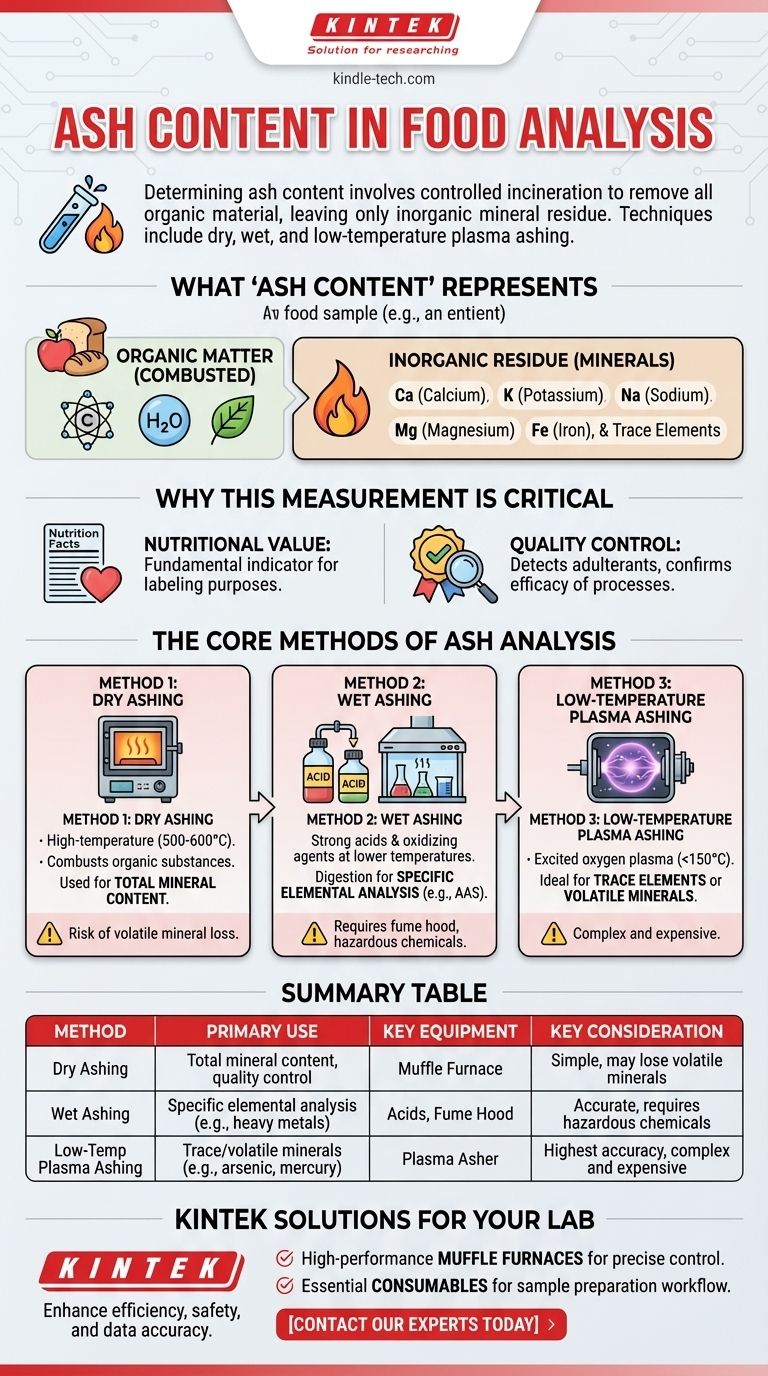
To determine the ash content of a food sample, the most common methods involve controlled incineration to burn off all organic material, leaving only the inorganic mineral residue. The primary techniques are dry ashing, wet ashing, and low-temperature plasma ashing, each selected based on the specific goals of the analysis.
Ash analysis is a foundational technique in food science for quantifying the total mineral content. The core principle is the complete removal of organic matter (carbon, hydrogen, nitrogen) through oxidation, which allows for the measurement of the noncombustible inorganic residue.

What "Ash Content" Actually Represents
Beyond Combustion: The Inorganic Residue
The term "ash" refers to the total amount of minerals present within a food. This residue is what remains after all water and organic material—like protein, fat, and carbohydrates—have been completely incinerated.
This inorganic residue consists of the oxides, sulfates, phosphates, and chlorides of essential minerals such as calcium, potassium, sodium, magnesium, and iron, as well as trace elements.
Why This Measurement is Critical
Measuring ash content is not just an academic exercise; it is a critical parameter in food quality and safety. It serves as a fundamental indicator of nutritional value, as it represents the food's total mineral content for labeling purposes.
Furthermore, it is a key metric in quality control. Unusually high or low ash content can indicate the presence of adulterants, such as sand in spices, or confirm the efficacy of processes like the milling of flour.
The Core Methods of Ash Analysis
The choice of method is dictated by the specific minerals being analyzed and the nature of the food sample itself.
Method 1: Dry Ashing
This is the most common and straightforward method. The food sample is placed in a high-temperature muffle furnace, typically at temperatures between 500 and 600°C.
The extreme heat combusts all organic substances, leaving behind a stable inorganic ash that can be weighed. This method is widely used for determining total mineral content.
Method 2: Wet Ashing
Wet ashing is primarily used when a sample needs to be prepared for the analysis of specific individual minerals, often using techniques like atomic absorption spectroscopy (AAS).
Instead of high heat, this method uses a combination of strong acids (like nitric and sulfuric acid) and oxidizing agents at lower temperatures to digest the organic matter. This keeps the minerals in a solution, ready for further analysis.
Method 3: Low-Temperature Plasma Ashing
This is a more specialized and gentle technique. It uses a vacuum chamber where oxygen is excited into a plasma state using electromagnetic radiation.
This excited oxygen effectively oxidizes the organic material at much lower temperatures (typically <150°C). This process is ideal for analyzing trace elements or volatile minerals (like mercury, lead, or arsenic) that might be lost at the high temperatures of a muffle furnace.
Understanding the Trade-offs
No single method is universally superior; each involves critical trade-offs that you must consider based on your analytical goals.
Accuracy vs. Simplicity
Dry ashing is simple, requires less hands-on time, and can handle larger sample batches. However, the high temperatures can cause the loss of volatile minerals, potentially underestimating their true concentration.
Wet ashing and low-temperature ashing are more complex and labor-intensive but provide a more accurate quantification of volatile or trace elements.
Safety and Equipment
Dry ashing requires a specialized high-temperature muffle furnace, which is a significant capital investment but is relatively safe to operate with standard precautions.
Wet ashing, on the other hand, involves highly corrosive acids and produces noxious fumes, mandating the use of a certified fume hood and rigorous safety protocols.
Making the Right Choice for Your Goal
Your objective dictates the appropriate method.
- If your primary focus is routine quality control or total mineral content for a nutrition label: Dry ashing is the most efficient and practical choice.
- If your primary focus is preparing a sample for specific elemental analysis (e.g., heavy metals or trace nutrients): Wet ashing is the standard procedure to prevent mineral loss and prepare a sample solution.
- If your primary focus is research involving highly volatile minerals in sensitive samples: Low-temperature plasma ashing offers the highest accuracy, though it is the most complex and expensive method.
Ultimately, selecting the correct ashing method is the first critical step in accurately characterizing the nutritional and quality profile of a food product.
Summary Table:
| Method | Primary Use | Key Equipment | Key Consideration |
|---|---|---|---|
| Dry Ashing | Total mineral content, quality control | Muffle Furnace | Simple, but may lose volatile minerals |
| Wet Ashing | Specific elemental analysis (e.g., heavy metals) | Acids, Fume Hood | Accurate, but requires hazardous chemicals |
| Low-Temp Plasma Ashing | Trace/volatile minerals (e.g., arsenic, mercury) | Plasma Asher | Highest accuracy, but complex and expensive |
Ready to Optimize Your Food Analysis Lab?
Choosing the right ashing method is critical for accurate nutritional labeling and quality control. KINTEK specializes in providing the reliable lab equipment you need to execute these methods precisely.
- For Dry Ashing: Our high-performance muffle furnaces offer the precise temperature control and uniform heating required for consistent results.
- For Sample Preparation: We supply essential consumables to support your entire workflow.
We understand the challenges of food science laboratories. Let us help you enhance your lab's efficiency, safety, and data accuracy.
Contact our experts today to discuss your specific needs and find the perfect solution for your lab.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What temperature do you fire alumina? Achieve Optimal Density and Strength
- Does ceramic break with heat? The Real Culprit is Thermal Shock
- Why refractory materials are used in furnaces? Ensure Safety, Efficiency, and Process Purity
- What are the precautions of muffle furnace? Essential Safety Protocols for Your Lab
- Why the melting temperature of ceramic is higher than for most metals? Unpacking Atomic Bond Strength



















