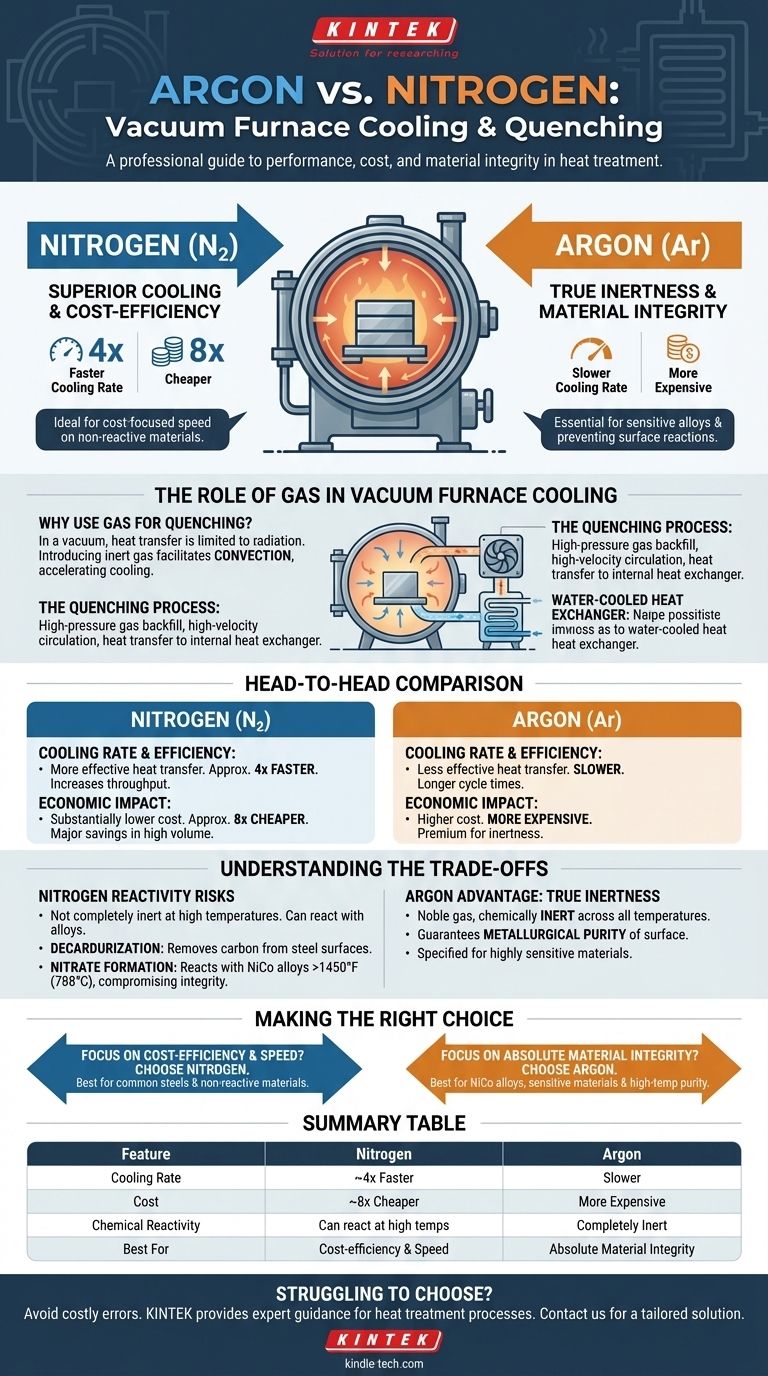
In a direct comparison, nitrogen is the superior cooling gas for vacuum furnaces in terms of both performance and cost. It offers a cooling rate approximately four times faster than argon and is about eight times cheaper, making it the default choice for a wide range of applications.
The decision between nitrogen and argon is a classic engineering trade-off. While nitrogen provides significant cost and speed advantages, argon's value lies in its complete chemical inertness, which is essential for preventing unwanted surface reactions with sensitive materials at high temperatures.

The Role of Gas in Vacuum Furnace Cooling
Why Use Gas for Quenching?
In a vacuum, heat transfer is limited primarily to radiation. To rapidly cool a workload—a process known as quenching—an inert gas is introduced back into the hot zone.
This gas facilitates convection, dramatically accelerating the transfer of heat from the workpiece to the furnace's cooling systems, such as water-cooled heat exchangers.
The Quenching Process
The process involves backfilling the heated furnace chamber with high-pressure gas. A powerful fan then circulates this gas, creating a high-velocity flow that moves heat away from the parts and toward the internal heat exchanger, which then transfers the heat out of the furnace.
A Head-to-Head Comparison: Nitrogen vs. Argon
Cooling Rate and Efficiency
Nitrogen's physical properties allow it to transfer heat more effectively than argon under typical furnace conditions. This results in a cooling rate that is approximately four times faster.
For processes where cycle time is a critical factor, the faster quenching provided by nitrogen can significantly increase throughput.
Economic Impact
The cost difference is substantial. Nitrogen is roughly eight times cheaper than argon, a factor that cannot be overlooked in production environments.
For high-volume heat-treating operations, the cumulative savings from using nitrogen can have a major impact on the bottom line.
Understanding the Trade-offs: When to Choose Argon
The Risk of Nitrogen Reactivity
The primary drawback of nitrogen is that it is not completely inert at the high temperatures seen in many heat-treating processes. It can react with certain elements in the alloys being treated.
This chemical reactivity is the single most important factor to consider when choosing between the two gases.
Impact on Steels
For some steels, nitrogen can have a slight decarburizing effect. This means it can react with and remove carbon from the surface of the part, potentially altering its final mechanical properties.
Impact on Nickel-Cobalt (NiCo) Alloys
At temperatures above 1450°F (788°C), nitrogen can react with the surface of NiCo alloys to form nitrates. This is often undesirable and can compromise the integrity and performance of the final component, especially in critical aerospace or medical applications.
Argon's Key Advantage: True Inertness
Argon is a noble gas, meaning it is chemically inert across the entire temperature range of a vacuum furnace. It will not react with the workpiece, no matter the material or temperature.
This absolute inertness guarantees the metallurgical purity of the part's surface, which is why it is specified for highly sensitive or reactive materials.
Making the Right Choice for Your Goal
- If your primary focus is cost-efficiency and speed: Nitrogen is the clear choice for treating common steels and other materials that are not susceptible to nitrogen reactions in your process window.
- If your primary focus is absolute material integrity: Argon is the only option when treating reactive materials like NiCo alloys or certain high-carbon steels at elevated temperatures where surface purity cannot be compromised.
Ultimately, the correct gas choice depends entirely on balancing the compelling economic benefits of nitrogen against the critical inertness your specific material requires.
Summary Table:
| Feature | Nitrogen | Argon |
|---|---|---|
| Cooling Rate | ~4x Faster | Slower |
| Cost | ~8x Cheaper | More Expensive |
| Chemical Reactivity | Can react with some alloys at high temps | Completely Inert |
| Best For | Cost-efficiency & speed on non-reactive materials | Absolute material integrity & sensitive alloys |
Struggling to choose the right quenching gas for your vacuum furnace process? The wrong choice can lead to costly rework, scrapped parts, or compromised material properties. KINTEK specializes in lab equipment and consumables, serving laboratory needs with expert guidance on heat treatment processes. Our team can help you analyze your specific materials and application to determine the optimal gas for maximizing throughput or ensuring flawless surface integrity.
Ensure your next heat treatment is a success. Contact our experts today to discuss your requirements and get a tailored solution from KINTEK.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the specific application of a vacuum oven in removing trace moisture from PEO? Ensure Battery Stability
- How is heat transferred through a gas or vacuum? Master the 3 Key Mechanisms
- What metals can be joined by brazing? Discover the Versatility of Modern Brazing Techniques
- What is a vacuum brazing furnace? Achieve Flawless, High-Strength Joining
- What is the maximum temperature of a heat treatment furnace? From 1100°C to 2200°C+
- What role does a high-vacuum furnace environment play in W-Cu combustion synthesis? Ensure Defect-Free Density
- How do vacuum furnaces heat? Achieve Purity and Precision in High-Temperature Processing
- What is the primary function of industrial ovens in lignocellulosic waste pretreatment? Maximize Energy Efficiency



















