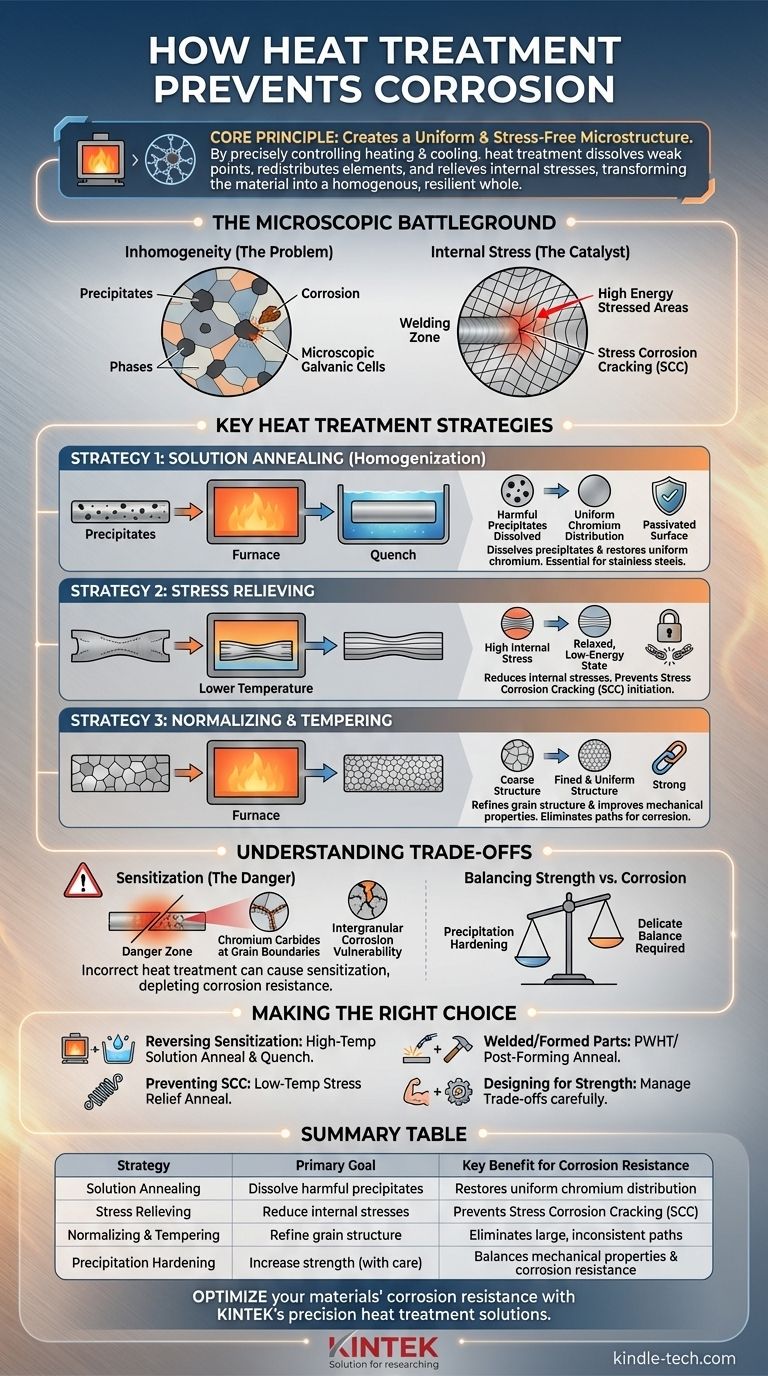
In essence, heat treatment prevents corrosion by creating a more uniform and stress-free microscopic structure within the metal. By precisely controlling heating and cooling cycles, you can dissolve weak points, redistribute protective elements, and relieve internal stresses that act as initiation sites for corrosive attack. This process transforms the material from a collection of vulnerable regions into a homogenous, resilient whole.
The core principle is not simply applying heat, but using a controlled thermal process to fundamentally alter a metal's microstructure. The goal is to eliminate the chemical and physical inconsistencies—like precipitates and internal stress—that make a material susceptible to corrosion.

The Microscopic Battleground: Why Metals Corrode
To understand how heat treatment works, you must first understand what makes a metal vulnerable. Corrosion rarely attacks a material uniformly; it seeks out and exploits microscopic inconsistencies.
The Problem of Inhomogeneity
Most high-performance alloys are not perfectly uniform. They can contain different phases or precipitates—tiny particles of a different chemical composition from the main body of the metal.
These precipitates can create microscopic galvanic cells. The area immediately surrounding the particle can become depleted of a key corrosion-resistant element (like chromium in stainless steel), making it anodic and highly susceptible to attack.
The Role of Internal Stress
Manufacturing processes like welding, forming, or machining introduce residual stress into the material. These stressed regions have higher internal energy.
This higher energy state makes the stressed areas more chemically reactive than the surrounding unstressed metal. This difference creates a pathway for specific, often catastrophic, corrosion mechanisms like Stress Corrosion Cracking (SCC).
Key Heat Treatment Strategies for Corrosion Control
Heat treatments are not one-size-fits-all. Each process is designed to solve a specific microstructural problem that leads to corrosion.
Strategy 1: Solution Annealing (Homogenization)
Solution annealing is the most powerful tool for combating corrosion caused by chemical inhomogeneity. It involves heating the alloy to a high temperature where the undesirable precipitates dissolve back into the metal matrix.
Think of it like dissolving sugar in water. At the right temperature, the clustered "sugar" (precipitates) dissolves and spreads evenly throughout the "water" (the metal matrix).
This process is critical for austenitic stainless steels. A rapid cooling or quench is then required to "freeze" this uniform state, preventing the harmful precipitates from reforming. This restores the even distribution of chromium, allowing the entire surface to form its protective passive layer.
Strategy 2: Stress Relieving
Stress relieving is a lower-temperature process designed specifically to reduce the internal stresses introduced during manufacturing.
The temperature is high enough to allow the metal's atoms to rearrange into a lower-energy, relaxed state, but not high enough to significantly alter its hardness or primary microstructure. This directly reduces the material's susceptibility to Stress Corrosion Cracking (SCC).
Strategy 3: Normalizing and Tempering
While primarily used to refine grain structure and improve mechanical properties, processes like normalizing and tempering can also enhance corrosion resistance.
By creating a finer, more uniform grain structure, these treatments eliminate the large, inconsistent microstructures that can provide easy paths for corrosion. A tempered structure in steel, for example, often provides better general corrosion resistance than a coarse, annealed one.
Understanding the Trade-offs: When Heat Treatment Can Go Wrong
Applying heat incorrectly is often worse than doing nothing at all. The wrong thermal cycle can actively create the very problems you are trying to solve.
The Danger of Sensitization
This is the most critical pitfall, especially for austenitic stainless steels. If a 300-series stainless steel is heated or slow-cooled through a specific temperature range (~450–850°C or 850–1550°F), the opposite of solution annealing occurs.
Chromium combines with carbon in the alloy to form chromium carbides along the grain boundaries. This process strips the chromium from the metal adjacent to the boundaries, leaving those zones depleted and extremely vulnerable to intergranular corrosion. A sensitized part can literally crumble at its grain boundaries when exposed to a corrosive environment.
Balancing Strength vs. Corrosion Resistance
Some heat treatments, like precipitation hardening (age hardening), are designed to increase strength by intentionally forming very fine precipitates.
While this dramatically improves mechanical properties, it's a delicate balance. If the process is not perfectly controlled (e.g., over-aging), the precipitates can grow too large or deplete the matrix of its protective elements, thereby reducing corrosion resistance.
Making the Right Choice for Your Application
The correct heat treatment is entirely dependent on the alloy, its condition, and the failure mode you are trying to prevent.
- If your primary focus is reversing sensitization in stainless steel: A high-temperature solution anneal followed by a rapid quench is the definitive solution to redissolve chromium carbides.
- If your primary focus is preventing Stress Corrosion Cracking (SCC): A low-temperature stress relief anneal is the correct choice to relax internal stresses without affecting the base metallurgy.
- If you are working with welded or cold-formed parts: Always consider if a Post-Weld Heat Treatment (PWHT) or post-forming anneal is required to restore the material's intended corrosion properties.
- If you are designing for maximum strength: Be acutely aware that the heat treatment used to achieve peak hardness may create trade-offs in corrosion resistance that require careful management.
Ultimately, using heat treatment for corrosion control is a precise act of metallurgical engineering designed to enforce uniformity at the microscopic level.
Summary Table:
| Heat Treatment Strategy | Primary Goal | Key Benefit for Corrosion Resistance |
|---|---|---|
| Solution Annealing | Dissolve harmful precipitates | Restores uniform chromium distribution for a stable passive layer |
| Stress Relieving | Reduce internal stresses | Prevents Stress Corrosion Cracking (SCC) initiation |
| Normalizing & Tempering | Refine grain structure | Eliminates large, inconsistent paths for corrosion |
| Precipitation Hardening | Increase strength (with care) | Balances mechanical properties with corrosion resistance |
Optimize your materials' corrosion resistance with precision heat treatment. KINTEK specializes in advanced lab furnaces and thermal processing equipment, helping laboratories and manufacturers achieve uniform, stress-free microstructures. Whether you're working with stainless steel, high-performance alloys, or welded components, our solutions ensure your materials meet the highest standards of durability and performance. Contact our experts today to discuss your specific application and discover the right heat treatment strategy for your needs.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the stages of melting metal? Mastering the 3-Step Process from Solid to Liquid
- What controls melting point? The Hierarchy of Forces from Ionic Bonds to Intermolecular Attractions
- Is sintering the same as welding? Key Differences in Material Bonding and Fusion Explained
- Which is better blast furnace or electric arc furnace? Choose the Right Steelmaking Technology for Your Needs
- What are sputtering systems used for? A Guide to Advanced Thin-Film Deposition
- What is the sintering process? A Guide to Manufacturing with Powdered Materials
- What is the significance of adding Polypropylene Carbonate (PPC) as a binder? Enhance Nickel-Alumina Structural Integrity
- Why is it necessary to configure efficient cold traps in membrane distillation? Ensure Flux Stability & Data Accuracy



















