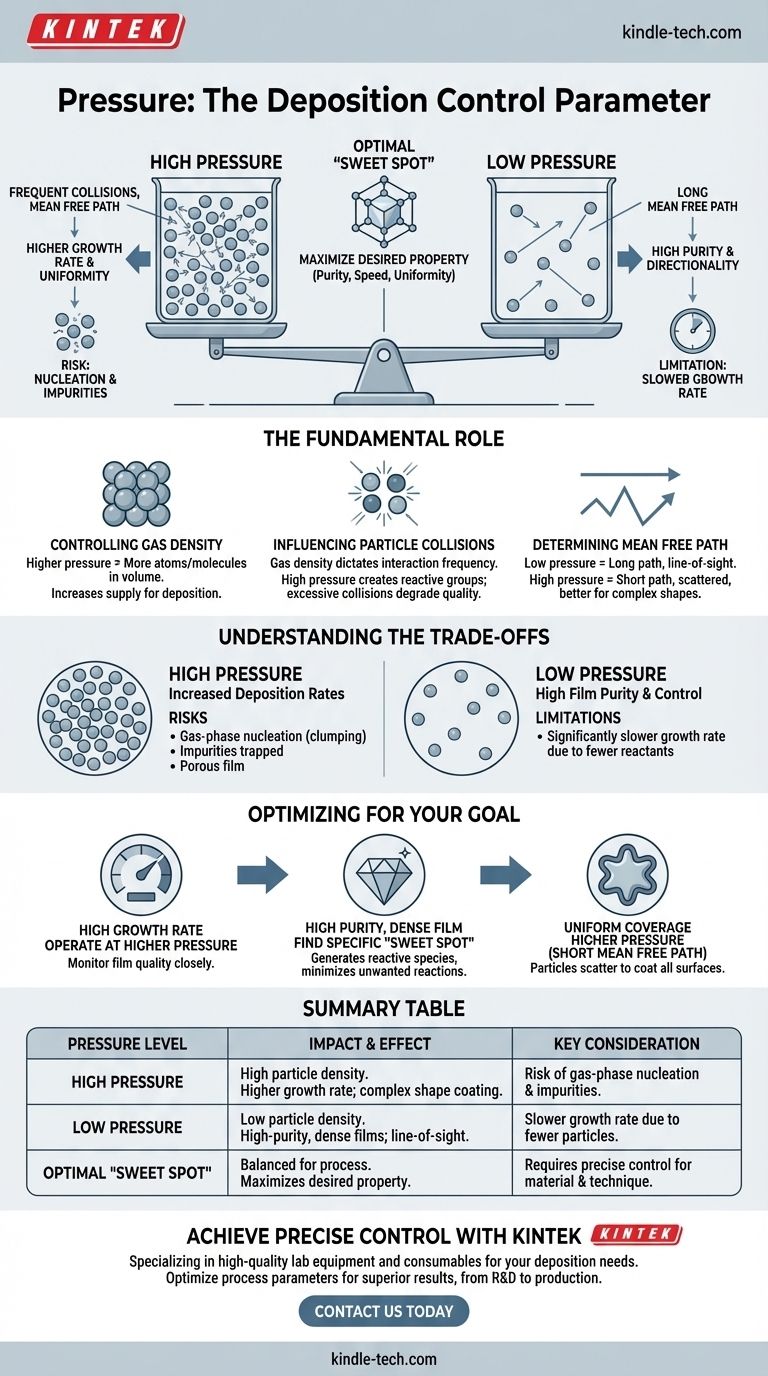
In deposition processes, pressure is one of the most critical control parameters. It directly dictates the concentration of gas particles within the deposition chamber and the frequency with which they collide, which in turn governs the growth rate, structure, and quality of the resulting film.
The core challenge is not simply raising or lowering pressure, but finding the precise optimal point. This "sweet spot" balances having enough reactive particles to ensure a high growth rate against preventing unwanted collisions that can degrade the quality of the final material.

The Fundamental Role of Pressure in Deposition
Understanding pressure is understanding how you control the behavior of the atoms and molecules that will eventually become your material. It's a primary lever for engineering the film's properties at the most basic level.
Controlling Gas Density
Pressure is a direct measure of the density of gas particles in the chamber.
A higher pressure means more atoms or molecules are packed into the same volume. This increases the supply of the material you are trying to deposit.
A lower pressure (closer to a vacuum) means fewer particles are present, creating a more rarefied environment.
Influencing Particle Collisions
Gas density directly impacts how often particles interact with each other before reaching the surface you're coating (the substrate).
At higher pressures, particles collide frequently. These collisions can be beneficial, as they can create the specific reactive atomic groups needed for deposition, like those required for growing high-quality diamond films.
However, excessive collisions can also cause particles to lose energy or react prematurely in the gas phase.
Determining the Mean Free Path
The mean free path is the average distance a particle can travel before colliding with another.
Low pressure creates a long mean free path. Particles travel in straight, uninterrupted lines from the source to the substrate, which is essential for "line-of-sight" deposition techniques.
High pressure results in a short mean free path. Particles follow a scattered, random path due to constant collisions, which can help coat complex shapes more uniformly.
Understanding the Trade-offs: The Pressure Balancing Act
Choosing a pressure setting is always an exercise in balancing competing factors. An optimal pressure for one goal, like speed, is often suboptimal for another, like quality.
The Risk of High Pressure
While higher pressure can increase deposition rates, it introduces significant risks. It can lead to gas-phase nucleation, where particles clump together before reaching the substrate, resulting in a porous or poor-quality film. It can also trap unwanted gas particles, creating impurities.
The Limitation of Low Pressure
Operating at very low pressure can ensure high film purity and a controlled deposition direction. However, this often comes at the cost of a significantly slower growth rate simply because there are fewer reactant particles available in the chamber at any given moment.
Optimizing Pressure for Your Deposition Goal
The "correct" pressure is entirely dependent on the material you are creating and the deposition technique you are using. The typical range for one process, like diamond deposition (several kPa to dozens of kPa), will be completely different for another.
- If your primary focus is a high growth rate: You will likely operate at a higher pressure to maximize the supply of reactant particles, but you must carefully monitor for declines in film quality.
- If your primary focus is a high-purity, dense film: You will need to find a specific pressure "sweet spot" that generates the necessary reactive species without causing unwanted gas-phase reactions.
- If your primary focus is uniform coverage on a complex shape: A higher pressure with a shorter mean free path may be necessary to ensure particles scatter and coat all surfaces, not just those in the direct line of sight.
Ultimately, pressure is the primary tool used to control the deposition environment and engineer the final properties of your material.
Summary Table:
| Pressure Level | Impact on Gas Density | Effect on Deposition | Key Consideration |
|---|---|---|---|
| High Pressure | High density of particles | Higher growth rate; better for coating complex shapes | Risk of gas-phase nucleation and impurities |
| Low Pressure | Low density of particles | High-purity, dense films; line-of-sight deposition | Slower growth rate due to fewer reactant particles |
| Optimal 'Sweet Spot' | Balanced for the specific process | Maximizes desired property (e.g., purity, speed, uniformity) | Requires precise control for the target material and technique |
Ready to achieve precise control over your deposition processes? The pressure settings in your system are critical for determining the growth rate, purity, and uniformity of your thin films. At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your specific deposition needs. Whether you are working on R&D or scaling up production, our expertise ensures you can optimize your process parameters for superior results.
Contact us today to discuss how our solutions can help you master your deposition environment and enhance your material outcomes!
Visual Guide
Related Products
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- What is the apparatus of chemical vapor deposition? The Essential Components for Thin Film Deposition
- What is the CVD method for synthetic diamonds? Grow Lab Diamonds from Gas with Precision
- Are CVD diamonds worth it? Unlock Brilliant Value & Ethical Clarity
- What is the construction and working of chemical vapor deposition? A Guide to High-Purity Thin Film Fabrication



















