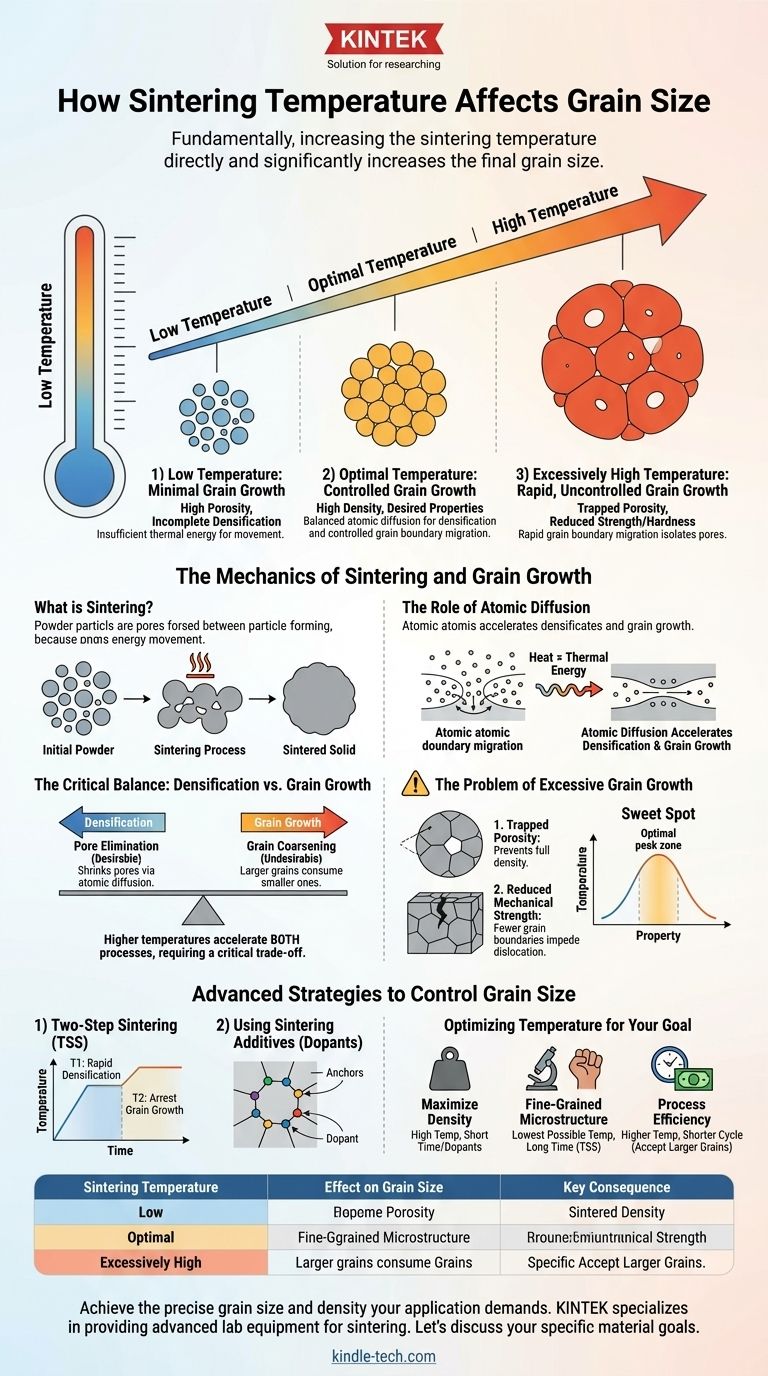
Fundamentally, increasing the sintering temperature directly and significantly increases the final grain size. This occurs because higher temperatures provide the thermal energy needed for atoms to move, a process called atomic diffusion. This enhanced atomic movement accelerates both the desirable process of densification (the removal of pores) and the often-undesirable process of grain growth, where smaller grains are consumed by larger ones.
The core challenge of sintering is not just reaching high density, but doing so while controlling grain size. Temperature is the primary lever for densification, but it simultaneously accelerates grain growth, forcing a critical trade-off that dictates the final properties of the material.

The Mechanics of Sintering and Grain Growth
To control grain size, you must first understand the fundamental forces at play during the sintering process. It is a competition between pore elimination and grain coarsening.
What is Sintering?
Sintering is a thermal treatment that bonds powder particles together into a solid, dense mass. The primary goal is to reduce or eliminate the empty space, or porosity, between the initial particles.
This process happens at temperatures below the material's melting point. Instead of melting and fusing, atoms move across the surfaces of particles to form and grow "necks" between them.
The Role of Atomic Diffusion
The engine driving this entire process is atomic diffusion. Temperature is the fuel. As you increase the temperature, you give atoms more kinetic energy, allowing them to move more freely and rapidly.
This movement allows atoms to migrate from areas of high stress (like the surface of a particle) to areas of lower stress (like the neck between two particles), causing the necks to grow and the pores to shrink.
How Grains Grow
A sintered material is composed of many individual crystals, or grains. The interface between any two grains is called a grain boundary.
Grain boundaries have higher energy than the interior of a grain. To minimize the total energy of the system, the material seeks to reduce its total grain boundary area. It achieves this through grain growth: larger grains, which are more energetically stable, consume their smaller neighbors.
Temperature as the Accelerator
Grain growth, like densification, is dependent on atomic diffusion. For a grain boundary to move and consume another grain, atoms must detach from one crystal lattice and reattach to the other.
Higher temperatures dramatically accelerate this atomic movement, leading to a much faster rate of grain boundary migration and, consequently, more rapid grain growth.
The Critical Balance: Densification vs. Grain Growth
The success of a sintering process is defined by how well it navigates the competition between achieving high density and preventing excessive grain growth.
Two Competing Processes
In the initial and intermediate stages of sintering, densification is often the dominant process. Pores are located at the grain boundaries, and atomic diffusion effectively shrinks them.
However, as the temperature rises or time extends, grain boundaries can break away from the pores. When a fast-moving grain boundary sweeps past a pore, that pore becomes trapped inside the grain, making it extremely difficult to remove.
The Problem of Excessive Grain Growth
Uncontrolled grain growth is often detrimental to the final material's performance. It can lead to two major problems:
- Trapped Porosity: As large grains grow rapidly, they can isolate pores within their interiors, preventing the material from ever reaching full density.
- Reduced Mechanical Strength: For most ceramics and metals, strength and hardness decrease as grain size increases. This is described by the Hall-Petch relationship, which states that smaller grains create more boundaries that impede dislocation movement, making the material stronger.
The "Sweet Spot" of Sintering
For any given material, there is an optimal temperature-time profile. Too low a temperature results in a porous, weak part. Too high a temperature creates a part with large, weak grains and potentially trapped porosity. The goal is to find the "sweet spot" that maximizes densification while keeping grain size within an acceptable range.
Advanced Strategies to Control Grain Size
Because the simple application of heat presents this trade-off, materials engineers have developed more sophisticated methods to decouple densification from grain growth.
Two-Step Sintering (TSS)
This method involves heating the material to a relatively high temperature (T1) to achieve a high rate of initial densification. Once the material reaches a critical density (typically >90%), the temperature is quickly lowered to a second, lower temperature (T2) and held.
At T2, the diffusion required for densification can still occur (especially to remove the final small pores), but the energy is too low for rapid grain boundary migration, effectively arresting grain growth.
Using Sintering Additives (Dopants)
Another powerful technique is the addition of small amounts of a second material, or dopant. These dopant ions tend to segregate to the grain boundaries.
This creates a "solute drag" effect, where the dopant atoms act like anchors that physically impede the movement of the grain boundary. This slows down grain growth, allowing densification to proceed to completion at higher temperatures without the penalty of extreme grain coarsening.
Optimizing Temperature for Your Goal
The ideal sintering temperature is not a single value; it is a parameter you must adjust based on your primary objective for the final component.
- If your primary focus is achieving maximum density: You must use a sufficiently high temperature to drive pore elimination, but consider a shorter hold time or the use of dopants to prevent runaway grain growth that could trap residual porosity.
- If your primary focus is a fine-grained microstructure (for strength and hardness): Prioritize the lowest possible temperature that can still achieve your target density, even if it requires a significantly longer sintering time. Advanced methods like two-step sintering are ideal for this goal.
- If your primary focus is process efficiency (cost and time): Higher temperatures and shorter cycle times are often favored. However, you must accept the resulting larger grain size and rigorously test that the material's mechanical properties still meet the application's requirements.
Ultimately, mastering the effect of temperature on grain size is the key to transforming a simple powder into a high-performance engineered component.
Summary Table:
| Sintering Temperature | Effect on Grain Size | Key Consequence |
|---|---|---|
| Low | Minimal Growth | High Porosity, Incomplete Densification |
| Optimal | Controlled Growth | High Density, Desired Mechanical Properties |
| Excessively High | Rapid, Uncontrolled Growth | Trapped Porosity, Reduced Strength/Hardness |
Achieve the precise grain size and density your application demands.
Controlling the sintering process is critical for developing materials with the right mechanical properties. KINTEK specializes in providing the advanced lab equipment and expert support you need to master this balance.
Our sintering furnaces offer precise temperature control and programmable profiles, enabling techniques like two-step sintering to achieve high density with fine grain size. Whether you're working on R&D or production, KINTEK's solutions help you optimize your process for strength, hardness, and efficiency.
Let's discuss your specific material goals. Contact our experts today to find the ideal sintering solution for your laboratory.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What are the disadvantages of nitriding over carburizing? A Guide to Process Limitations
- Why is a vacuum oven necessary for drying NVOPF electrode sheets? Ensure Battery Stability and Purity
- Can an arc occur in a vacuum? Uncover the Hidden Dangers of High-Voltage Vacuum Systems
- What is the application of vacuum mold casting? Achieve Rapid, High-Fidelity Prototyping and Bridge-to-Production
- How does the use of a vacuum oven benefit cellulose/MoS2 composites? Enhance Material Integrity and Performance
- What are the disadvantages of pyrolysis of plastic waste? Key Economic and Technical Hurdles
- What role does a vacuum diffusion welding furnace play in the fabrication of multi-layer titanium alloy laminates?
- How do gas nozzles facilitate energy recycling in activation furnaces? Enhance Efficiency in Activated Carbon Production



















