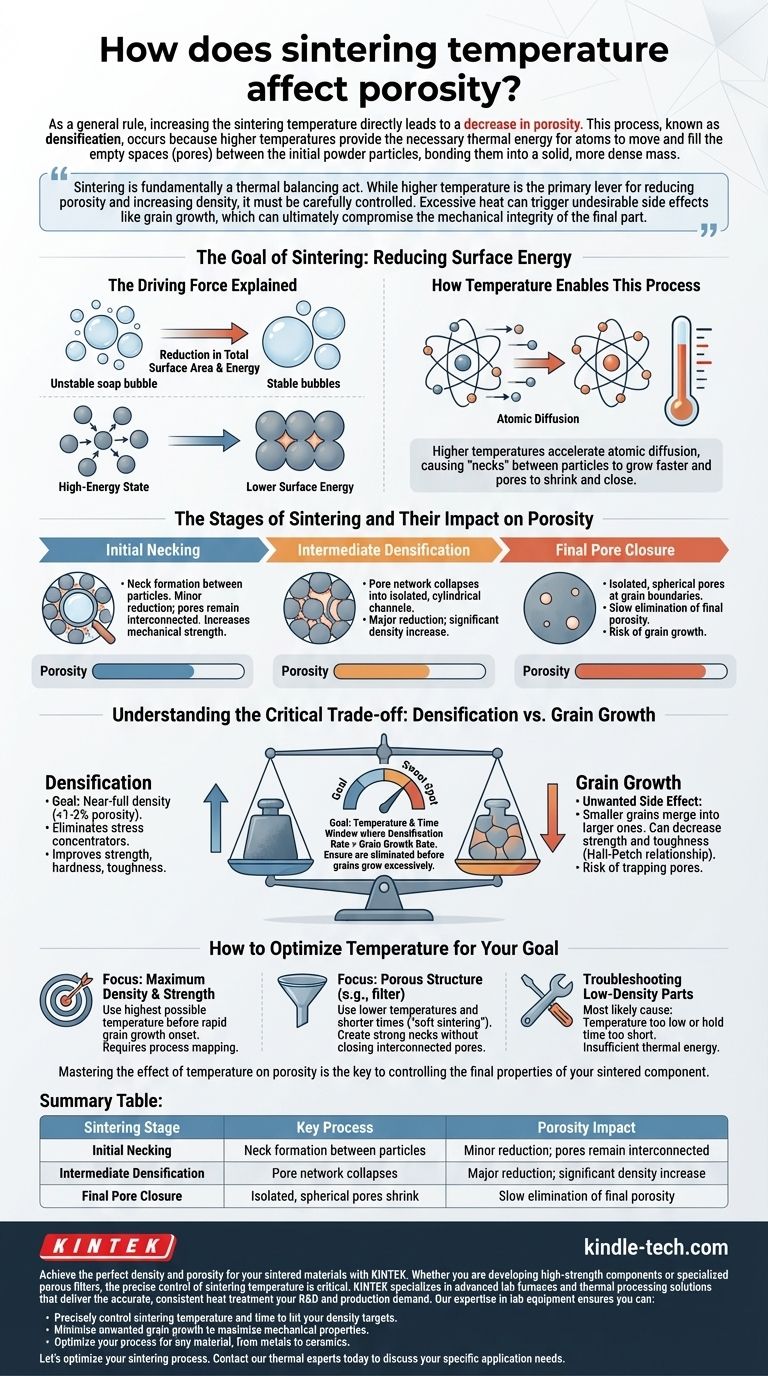
As a general rule, increasing the sintering temperature directly leads to a decrease in porosity. This process, known as densification, occurs because higher temperatures provide the necessary thermal energy for atoms to move and fill the empty spaces (pores) between the initial powder particles, bonding them into a solid, more dense mass.
Sintering is fundamentally a thermal balancing act. While higher temperature is the primary lever for reducing porosity and increasing density, it must be carefully controlled. Excessive heat can trigger undesirable side effects like grain growth, which can ultimately compromise the mechanical integrity of the final part.

The Goal of Sintering: Reducing Surface Energy
The Driving Force Explained
Imagine a collection of soap bubbles. Over time, smaller bubbles will merge to form larger ones because this configuration has less total surface area and is more energetically stable. Powder particles in a green body behave similarly.
The vast number of individual particles creates an enormous amount of surface area, which is a high-energy state. Sintering is the process by which the material reduces this total surface energy by bonding particles together and eliminating the voids between them.
How Temperature Enables This Process
This reduction in surface energy is not spontaneous; it requires energy to get started. Sintering temperature provides the activation energy needed for atomic diffusion—the movement of atoms.
Atoms migrate from the bulk of the particles to the points of contact and into the pores. Higher temperatures dramatically accelerate this diffusion, causing the "necks" between particles to grow faster and the pores to shrink and eventually close.
The Stages of Sintering and Their Impact on Porosity
The effect of temperature on porosity is not linear. It occurs across three distinct, often overlapping, stages.
Stage 1: Initial Necking
In the early stage, at lower temperatures, the primary event is the formation and growth of "necks" at the contact points between adjacent particles.
This initial bonding significantly increases the mechanical strength of the component, but it causes only a minor reduction in overall porosity. The pores are still an interconnected network.
Stage 2: Intermediate Densification
As the temperature rises further, the process enters the intermediate stage. This is where the most significant densification occurs.
The interconnected pore network begins to collapse into more isolated, cylindrical channels. The rapid shrinkage of these channels accounts for the majority of porosity reduction, leading to a substantial increase in the part's density.
Stage 3: Final Pore Closure
In the final stage, the remaining porosity consists of isolated, spherical pores, typically located at the boundaries between the crystalline grains.
Eliminating these last few percent of pores is slow and requires the highest temperatures. It's in this stage that the risk of other temperature-driven effects becomes most critical.
Understanding the Critical Trade-off: Densification vs. Grain Growth
Simply using the highest possible temperature is not always the best strategy. The most important factor to manage is the competition between densification and grain growth.
Why Densification is the Goal
For most structural applications, the goal is to achieve near-full density (less than 1-2% porosity). Pores act as stress concentrators and crack initiation sites. Eliminating them dramatically improves properties like strength, hardness, and fracture toughness.
The Unwanted Side Effect: Grain Growth
Unfortunately, the same thermal energy that drives densification also drives grain growth. This is a process where smaller crystal grains within the material merge to become larger ones.
Excessive grain growth is often detrimental, as it can lead to a decrease in strength and toughness according to principles like the Hall-Petch relationship. If grains grow too large before the pores are eliminated, pores can become trapped within the grains, making them nearly impossible to remove.
Finding the Optimal "Sweet Spot"
The goal of a successful sintering cycle is to keep the material in a temperature and time window where the rate of densification is much faster than the rate of grain growth. This ensures pores are eliminated before the grains become excessively large.
How to Optimize Temperature for Your Goal
Temperature is the most powerful variable, but it does not act alone. Achieving your desired porosity requires considering the entire system. A successful outcome depends on balancing temperature with time and understanding your starting material.
- If your primary focus is maximum density and strength: Your strategy is to use the highest possible temperature that allows for pore closure before the onset of rapid grain growth. This often involves careful process mapping and material characterization.
- If your primary focus is a porous structure (e.g., a filter): You should use lower temperatures and shorter times, a process often called "soft sintering." The goal is only to create strong necks between particles (Stage 1) without significantly closing the interconnected pore network.
- If you are troubleshooting low-density parts: The most likely cause is that your sintering temperature is too low or your hold time is too short. The material simply did not receive enough thermal energy to complete the densification process.
Mastering the effect of temperature on porosity is the key to controlling the final properties of your sintered component.
Summary Table:
| Sintering Stage | Key Process | Porosity Impact |
|---|---|---|
| Initial Necking | Neck formation between particles | Minor reduction; pores remain interconnected |
| Intermediate Densification | Pore network collapses | Major reduction; significant density increase |
| Final Pore Closure | Isolated, spherical pores shrink | Slow elimination of final porosity |
Achieve the perfect density and porosity for your sintered materials with KINTEK.
Whether you are developing high-strength components or specialized porous filters, the precise control of sintering temperature is critical. KINTEK specializes in advanced lab furnaces and thermal processing solutions that deliver the accurate, consistent heat treatment your R&D and production demand.
Our expertise in lab equipment ensures you can:
- Precisely control sintering temperature and time to hit your density targets.
- Minimize unwanted grain growth to maximize mechanical properties.
- Optimize your process for any material, from metals to ceramics.
Let's optimize your sintering process. Contact our thermal experts today to discuss your specific application needs.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Spark Plasma Sintering Furnace SPS Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- Can you change the color of zirconia crowns? Understanding the Permanent Nature of Zirconia
- What are the white spots on zirconia after sintering? A Guide to Diagnosing and Preventing Defects
- What is one of the newest applications for dental ceramics? Monolithic Zirconia for Full-Arch Bridges
- What is the effect of zirconia sintering temperature? Master the Key to Strength and Stability
- What is the price of zirconia sintering furnace? Invest in Precision, Not Just a Price Tag



















