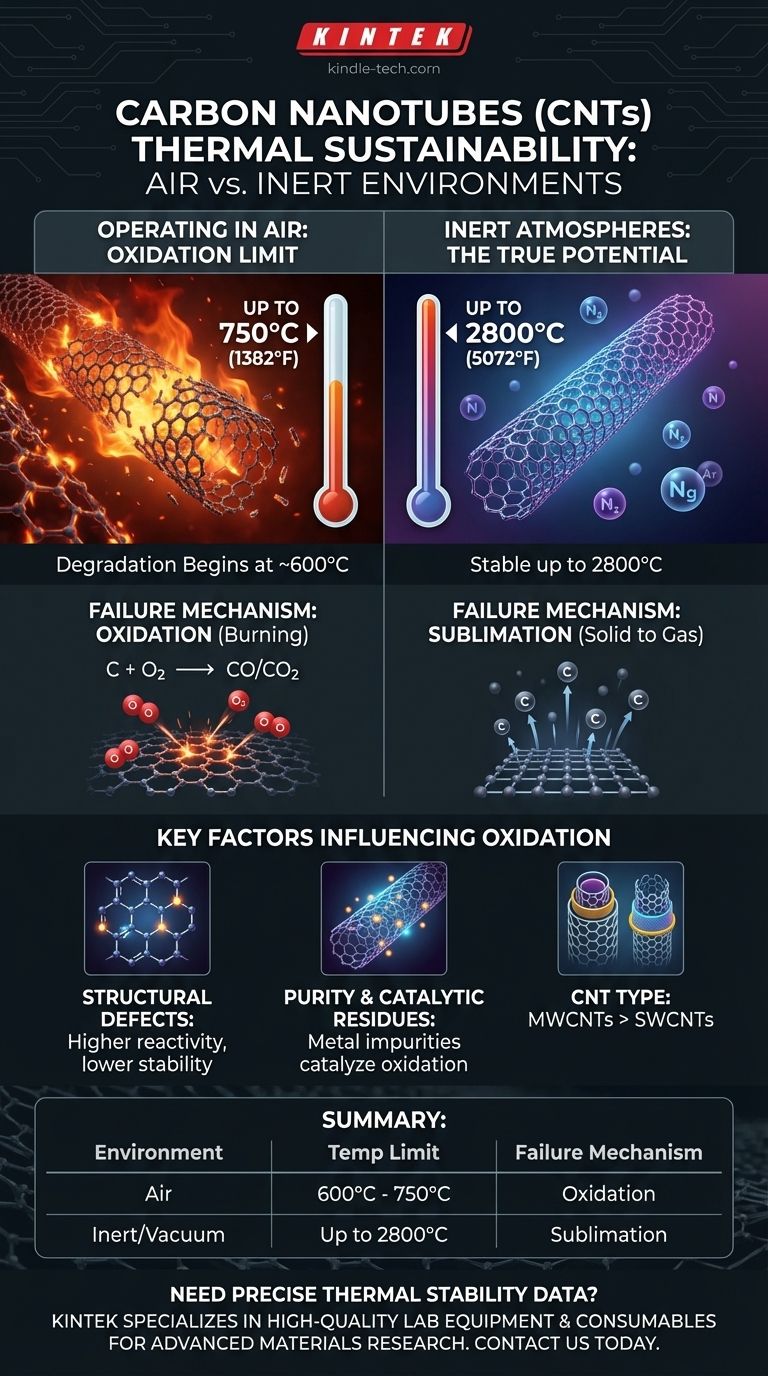
In an air environment, high-quality carbon nanotubes (CNTs) can typically sustain temperatures up to approximately 750°C (1382°F). However, this is not a fixed ceiling. The actual temperature limit is highly dependent on the structural quality, purity, and type of the nanotubes, with degradation often beginning at temperatures as low as 600°C. The primary failure mechanism is not melting but oxidation, where the carbon atoms react with atmospheric oxygen.
The thermal stability of a carbon nanotube is not an intrinsic property but is overwhelmingly defined by its environment. While CNTs can withstand extreme heat (over 2000°C) in a vacuum or inert gas, their practical temperature limit in air is dictated by their oxidation threshold, which is influenced by their structural integrity and purity.

The Critical Role of the Environment
Understanding why the operating atmosphere is the single most important factor is key to correctly applying CNTs in any high-temperature scenario.
Operation in Air: The Oxidation Limit
In the presence of oxygen, the strong carbon-carbon bonds that give CNTs their strength become vulnerable at high temperatures. This reaction begins to accelerate significantly in the 600°C to 750°C range.
The process involves oxygen molecules breaking down the graphitic structure of the nanotube walls, converting the carbon into carbon monoxide (CO) and carbon dioxide (CO2) gas. This effectively "burns" the nanotubes away, leading to a loss of structural and electrical properties.
Operation in Inert Atmospheres: The True Potential
When oxygen is removed, the performance of CNTs changes dramatically. In a vacuum or an inert gas environment (such as argon or nitrogen), carbon nanotubes are stable up to 2800°C (5072°F).
At this extreme temperature, they do not oxidize. Instead, they eventually fail through sublimation, where the solid carbon turns directly into a gas. This demonstrates that the material's intrinsic thermal stability is exceptionally high.
Key Factors Influencing Oxidation Temperature
Not all CNTs are created equal. Several factors determine the precise temperature at which oxidation begins, creating the wide stability range seen in practice.
Structural Defects
The ideal nanotube has a perfect hexagonal lattice of carbon atoms. However, real-world CNTs contain defects like vacancies (missing atoms) or other atomic arrangements. These defect sites are points of higher energy and are more chemically reactive, serving as initiation points for oxidation. Fewer defects mean a higher degradation temperature.
Purity and Catalytic Residues
CNTs are often synthesized using metal catalyst particles (e.g., iron, nickel, cobalt). If these metallic impurities are not fully removed during purification, they can remain in the final product.
These residual metal particles can catalyze the oxidation of carbon, actively lowering the temperature at which the nanotubes begin to break down. High-purity CNTs will always exhibit superior thermal stability in air.
CNT Type: SWCNTs vs. MWCNTs
Multi-walled carbon nanotubes (MWCNTs) generally have higher thermal stability in air than single-walled carbon nanotubes (SWCNTs).
This is because the outer walls of an MWCNT can oxidize first, effectively shielding the inner, protected walls. This layered structure provides a degree of sacrificial protection, preserving the nanotube's integrity for a longer duration at elevated temperatures.
Understanding the Trade-offs and Degradation Process
It is crucial to recognize that the thermal failure of CNTs is not an instantaneous event, which has significant implications for engineering design.
Gradual vs. Abrupt Failure
Oxidation does not begin suddenly at a specific temperature. It is a gradual process that accelerates as the temperature rises. This means a material containing CNTs may begin to lose performance well before catastrophic failure, a factor that must be accounted for in safety margins.
The Impact of Amorphous Carbon
Many raw CNT products contain a significant amount of amorphous carbon (soot) as a byproduct of synthesis. This non-crystalline carbon is less stable and will oxidize at a much lower temperature (often 300-400°C) than the nanotubes themselves. This can cause initial mass loss in an analysis that might be mistaken for the CNTs degrading.
The Misconception of a Single Limit
Engineers must avoid designing around a single, absolute temperature limit for CNTs in air. The 750°C figure should be considered an upper bound for ideal materials, not a reliable operating temperature for all applications. Real-world performance will almost always be lower.
How to Apply This to Your Project
Your design strategy must be guided by your specific operating conditions and material quality.
- If your primary focus is operating in an open-air system: Assume a conservative upper limit of 600°C and source the highest-purity, lowest-defect MWCNTs available to maximize stability.
- If your primary focus is pushing absolute thermal limits: You must operate in a vacuum or an inert gas environment; this is the only way to unlock the intrinsic stability of CNTs above 2000°C.
- If your primary focus is using CNTs as a composite additive: Recognize that the polymer or ceramic matrix will almost certainly be the limiting factor, as most matrix materials degrade at temperatures far below the oxidation point of the CNTs themselves.
By understanding that the environment—not intrinsic heat tolerance—is the primary constraint, you can accurately design systems that leverage the remarkable properties of carbon nanotubes.
Summary Table:
| Environment | Approximate Temperature Limit | Key Failure Mechanism |
|---|---|---|
| Air (Oxygen Present) | 600°C - 750°C (1112°F - 1382°F) | Oxidation (Burning) |
| Inert Gas / Vacuum | Up to 2800°C (5072°F) | Sublimation |
| Factor | Impact on Thermal Stability in Air |
|---|---|
| Structural Defects | More defects lower oxidation temperature |
| Purity (Catalytic Residues) | Metal impurities catalyze oxidation, lowering stability |
| CNT Type (SWCNT vs. MWCNT) | MWCNTs generally more stable due to sacrificial outer walls |
Need precise thermal stability data for your carbon nanotube application?
KINTEK specializes in providing high-quality lab equipment and consumables tailored for advanced materials research. Whether you're working with carbon nanotubes in controlled atmospheres or require precise temperature management tools, our solutions ensure accurate and reliable results.
Contact our experts today to discuss how we can support your laboratory's specific needs and help you achieve superior performance in your high-temperature experiments.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What is adhesion in thin film? The Key to Preventing Coating Failure
- Are carbon nanotubes safe for the environment? Weighing Performance Benefits Against Ecological Risks
- What is the effect of deposition rate on thin film? Control Microstructure for Better Performance
- What materials are used in thin film semiconductors? A Guide to Engineering High-Performance Layers
- What are the methods of graphene production? Top-Down vs. Bottom-Up for Your Lab's Needs
- What is the process of sputtering chemically? Master Reactive Sputtering for Superior Thin Films
- What is the coating material for glass? Select the Right Functional Layer for Your Needs
- What are the industrial applications for Silver coatings? Maximize Optical, Electrical, and Catalytic Performance



















