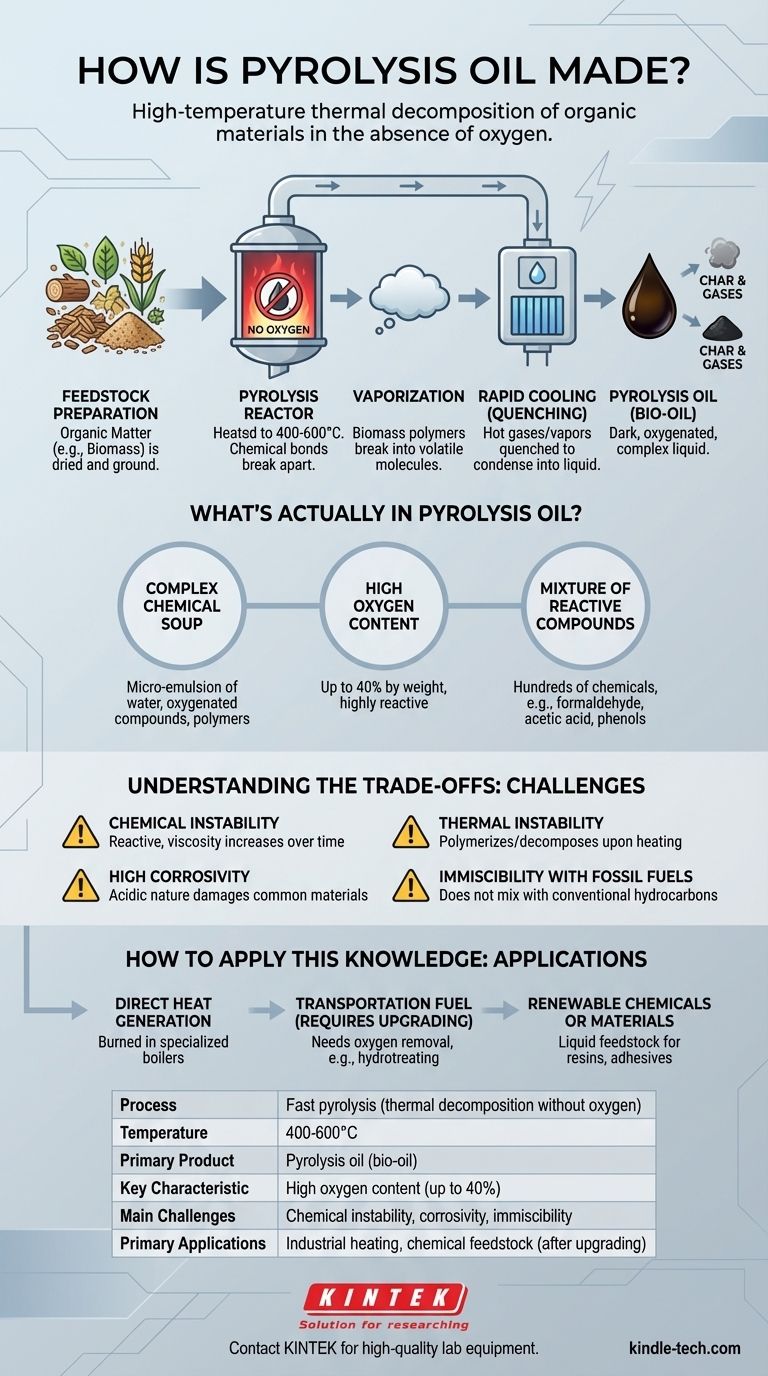
Pyrolysis oil is created through a process of high-temperature thermal decomposition in the absence of oxygen. This process, known as fast pyrolysis, rapidly breaks down organic materials like biomass into a vapor. These hot vapors are then quickly cooled and condensed into a dark, viscous liquid emulsion officially called pyrolysis oil, but also known as bio-oil or biocrude.
The core concept is not simply melting organic matter, but chemically decomposing it with intense heat in an oxygen-starved environment. The resulting liquid is not a true oil like petroleum, but a complex, unstable, and highly oxygenated intermediate product that presents significant challenges alongside its potential.

The Core Process: From Biomass to Bio-Oil
Pyrolysis is a carefully controlled thermal reaction that deconstructs organic material into three primary products: the liquid bio-oil, non-condensable gases (syngas), and a solid char. The yield of each depends on the precise process conditions.
The Feedstock: Any Organic Matter
The process begins with an organic feedstock, most commonly biomass such as wood, agricultural waste, or even specialized crops. This material is typically dried and ground into small particles to ensure rapid heat transfer.
The Key Ingredients: Heat and No Oxygen
The feedstock is fed into a reactor and heated to extreme temperatures (typically 400-600°C) in a near-zero oxygen environment. The absence of oxygen is critical; it prevents the material from simply burning (combustion) and instead forces its chemical bonds to break apart.
The Transformation: From Solid to Vapor
This intense, oxygen-free heat causes the large organic polymers in the biomass (like cellulose and lignin) to vaporize and break down into a wide range of smaller, volatile molecules. This all happens in a matter of seconds.
The Final Step: Rapid Cooling (Quenching)
These hot gases and vapors are immediately removed from the reactor and rapidly cooled, or "quenched." This rapid condensation freezes the chemical reactions in place, capturing a wide array of compounds in a liquid state. This liquid is the final pyrolysis oil.
What's Actually in Pyrolysis Oil?
Understanding the composition of bio-oil is essential to understanding its behavior. It is fundamentally different from crude oil derived from fossils.
A Complex Chemical Soup
Pyrolysis oil is a micro-emulsion composed of water, oxygenated organic compounds, and polymers derived from the original biomass. It is a dense, acidic liquid with a pungent, smoky odor.
The Defining Feature: High Oxygen Content
The most critical characteristic of bio-oil is its high oxygen content, which can be up to 40% by weight. This oxygen is bound within the molecular structure of the various chemical compounds.
A Mixture of Reactive Compounds
The oil is not one substance but a complex mixture of hundreds of different chemicals. This includes everything from simple, low molecular weight compounds like formaldehyde and acetic acid to larger, more complex molecules like phenols and oligosaccharides.
Understanding the Trade-offs: The Challenges of Bio-Oil
The unique chemical makeup of pyrolysis oil makes it a difficult substance to handle, store, and use without further processing. Its high oxygen content is the root cause of most of its limitations.
Chemical Instability
Bio-oil is composed of reactive, intermediate products. Over time, it is not stable. The compounds within it continue to react, causing a gradual increase in viscosity and potentially leading to phase separation.
Thermal Instability
Heating the oil can accelerate these unwanted reactions. When heated to around 100°C or more, the oil can rapidly polymerize, producing a solid residue and releasing volatile organic compounds.
High Corrosivity
The presence of organic acids, primarily acetic acid, makes the oil highly corrosive to common construction materials like carbon steel. This requires specialized, more expensive equipment for storage and transportation.
Immiscibility with Fossil Fuels
Due to its high oxygen content and polar nature, pyrolysis oil does not mix with conventional hydrocarbon fuels like diesel or heating oil. This prevents simple blending and requires either dedicated combustion systems or significant upgrading.
How to Apply This Knowledge
The primary challenge and opportunity with pyrolysis oil revolves around managing or removing its high oxygen content. This reality dictates its practical applications.
- If your primary focus is direct heat generation: Bio-oil can be burned in specialized industrial boilers and furnaces, but the equipment must be designed to handle its high viscosity, corrosivity, and different combustion properties.
- If your primary focus is producing a drop-in transportation fuel: Raw pyrolysis oil is entirely unsuitable. It requires an intensive secondary upgrading process (like hydrotreating) to remove oxygen, which adds significant cost and complexity.
- If your primary focus is creating renewable chemicals or materials: The oil can be seen as a liquid feedstock. Its rich mixture of phenols and other compounds can be extracted for use in products like resins, adhesives, or plastics, but this requires advanced refining.
Ultimately, viewing pyrolysis oil as a reactive, oxygen-rich chemical intermediate—not a finished fuel—is the key to evaluating its true potential for any project.
Summary Table:
| Aspect | Key Details |
|---|---|
| Process | Fast pyrolysis (thermal decomposition without oxygen) |
| Temperature | 400-600°C |
| Primary Product | Pyrolysis oil (bio-oil) |
| Key Characteristic | High oxygen content (up to 40%) |
| Main Challenges | Chemical instability, corrosivity, immiscibility with fossil fuels |
| Primary Applications | Industrial heating, chemical feedstock (after upgrading) |
Ready to source reliable equipment for your pyrolysis or biomass conversion research? KINTEK specializes in high-quality lab equipment and consumables for advanced thermal processes. Whether you're developing new bio-oil upgrading techniques or optimizing reactor conditions, our products support precision, safety, and efficiency. Contact our experts today to discuss how we can equip your laboratory for success in renewable energy innovation.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















