In short, determining ash content involves weighing a sample, heating it in a muffle furnace to a high temperature to completely burn off all organic matter, and then weighing the remaining inorganic residue. This simple "loss-on-ignition" process is a fundamental method for quantifying the non-combustible mineral content within a material.
The core principle is gravimetric analysis: by carefully measuring the mass lost during high-temperature combustion, you can precisely calculate the percentage of inorganic, non-combustible material (ash) that remains. Accuracy hinges on complete combustion and preventing moisture contamination during cooling.

The Principle: What is Ash?
Ash content analysis operates on a straightforward concept known as thermogravimetric analysis, or more simply, "loss-on-ignition."
Defining "Ash"
Ash is the inorganic residue that remains after a sample is completely burned. It consists of mineral components like oxides, silicates, and phosphates that do not combust at the high temperatures used in the analysis.
The Combustion Process
When a sample is heated in a muffle furnace, typically to temperatures between 550°C and 900°C, all organic components (carbon-based compounds) react with oxygen and are converted into gaseous products like carbon dioxide and water vapor. These gases are vented, leaving only the stable, inorganic minerals behind.
The Step-by-Step Procedure for Ashing
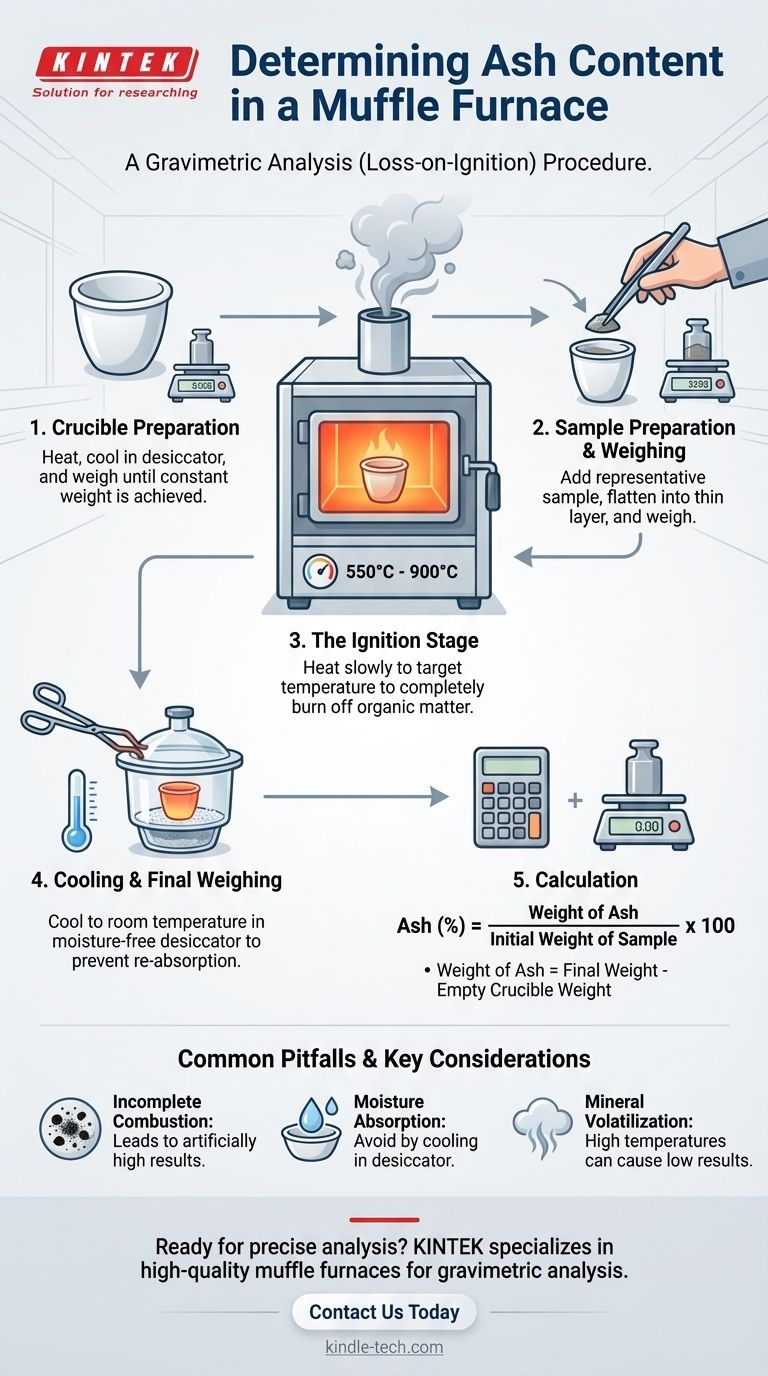
A reliable ash content determination follows a precise and repeatable procedure. Each step is designed to eliminate variables and ensure an accurate final measurement.
1. Crucible Preparation
Before introducing your sample, the container itself must be prepared. An empty porcelain or platinum crucible is heated in the muffle furnace at the testing temperature (e.g., 900°C) for about an hour.
It is then removed with tongs, placed in a desiccator to cool to room temperature, and weighed precisely. This process is repeated until the crucible achieves a constant weight, ensuring any moisture or residue is removed.
2. Sample Preparation and Initial Weighing
A representative portion of the material is placed into the pre-weighed crucible. For solids like polymers or rubber, the sample should be flattened into a thin layer. This maximizes surface area and ensures uniform, complete combustion.
The crucible with the sample is then weighed again. The initial sample weight is calculated by subtracting the constant weight of the empty crucible.
3. The Ignition Stage
The crucible containing the sample is placed in the cool or slightly warm muffle furnace. The furnace is then programmed to ramp up to the target temperature slowly to prevent the sample from sputtering and losing mass.
The sample is "ashed" at this peak temperature (e.g., 900°C) for several hours until all the black, carbonaceous material is gone, leaving a light-colored ash.
4. Cooling and Final Weighing
Using long tongs and wearing heat-resistant gloves, the hot crucible is carefully removed from the furnace and immediately placed inside a desiccator.
A desiccator is a sealed container with a drying agent that provides a moisture-free environment. This is critical because hot ash is hygroscopic and will absorb atmospheric moisture as it cools, artificially increasing its weight.
Once the crucible has cooled completely to room temperature, it is weighed one last time.
5. Calculation
The percentage of ash is calculated using a simple formula:
Ash (%) = (Weight of Ash / Initial Weight of Sample) x 100
Where:
- Weight of Ash = (Weight of crucible + ash) - (Weight of empty crucible)
- Initial Weight of Sample = (Weight of crucible + sample) - (Weight of empty crucible)
Common Pitfalls and Key Considerations
While the procedure is simple, several factors can compromise the accuracy of your results. Awareness of these issues is the mark of a skilled technician.
Incomplete Combustion
If the sample is too thick or the ashing time is too short, some organic material may not fully combust. This leaves carbon residue (seen as black specks) mixed with the ash, leading to an artificially high ash content reading.
Moisture Absorption
Failing to use a desiccator for cooling is the most common error. A warm crucible creates convection currents, and the ash itself will readily pull moisture from the air, skewing the final weight upward. Always cool to ambient temperature inside a desiccator.
Volatilization of Minerals
Some inorganic salts can become volatile and vaporize at very high temperatures. If the ashing temperature is too high for the specific minerals in your sample, you can lose some of the ash, leading to an artificially low result. This is why standardized methods (like ASTM) specify precise temperatures for different materials.
Safety and Equipment Care
Always handle hot crucibles with tongs and wear appropriate personal protective equipment (PPE), including heat-resistant gloves and safety glasses. After the analysis is complete, ensure the furnace's power supply is turned off and the equipment is maintained according to manufacturer guidelines.
Applying This to Your Goal
Your specific objective determines which parts of the process to emphasize.
- If your primary focus is routine quality control: Consistency is paramount. Strictly follow a validated Standard Operating Procedure (SOP), paying close attention to achieving constant crucible weight and using identical ashing times and temperatures for every test.
- If your primary focus is material characterization or R&D: Understanding the method's limitations is key. Be aware that the results represent total inorganic filler, not its specific composition, and consider the potential for mineral volatilization at your chosen temperature.
- If your primary focus is troubleshooting or failure analysis: Look for deviations from the expected ash content. An unusually high value might indicate incomplete combustion or contamination, while a low value could point to an incorrect material formulation.
By mastering this procedure, you gain a clear and reliable window into the fundamental composition of your material.
Summary Table:
| Key Step | Purpose | Critical Consideration |
|---|---|---|
| Crucible Preparation | Achieve constant weight | Pre-heat to remove moisture and residue |
| Sample Weighing | Measure initial mass | Flatten sample for uniform combustion |
| Ignition | Burn off organic matter | Control temperature ramp to prevent sputtering |
| Cooling | Prevent moisture absorption | Use desiccator for hygroscopic ash |
| Final Weighing | Calculate ash percentage | Ensure complete cooling to room temperature |
Ready to achieve precise ash content analysis in your lab?
KINTEK specializes in high-quality muffle furnaces and lab equipment designed for accurate gravimetric analysis. Our solutions ensure complete combustion, uniform heating, and reliable results for your quality control, R&D, or material characterization needs.
Contact us today to find the perfect furnace for your application and elevate your analytical capabilities.
Get in touch with our experts now!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What temperature is needed for sintering pottery? A Guide to Perfect Firing for Durability
- What is the role of a high-temperature sintering furnace in LATP synthesis? Unlock NASICON Phase Purity
- What is the difference between muffle furnace and hot oven? A Guide to Choosing the Right Thermal Tool
- How does a ceramic high-temperature furnace ensure experimental validity? Stabilize 100-Hour Molten Salt Corrosion Tests
- Why is a precision laboratory oven required for zirconium dioxide nanoparticle synthesis? Master Structural Integrity
- What is the temperature range of a muffle furnace? From 1100°C to 1800°C Based on Heating Elements
- What role does a programmable muffle furnace play in studying the high-temperature performance of geopolymers?
- What is the use of muffle furnace in food laboratory? Essential for Accurate Nutritional Analysis & Quality Control



















