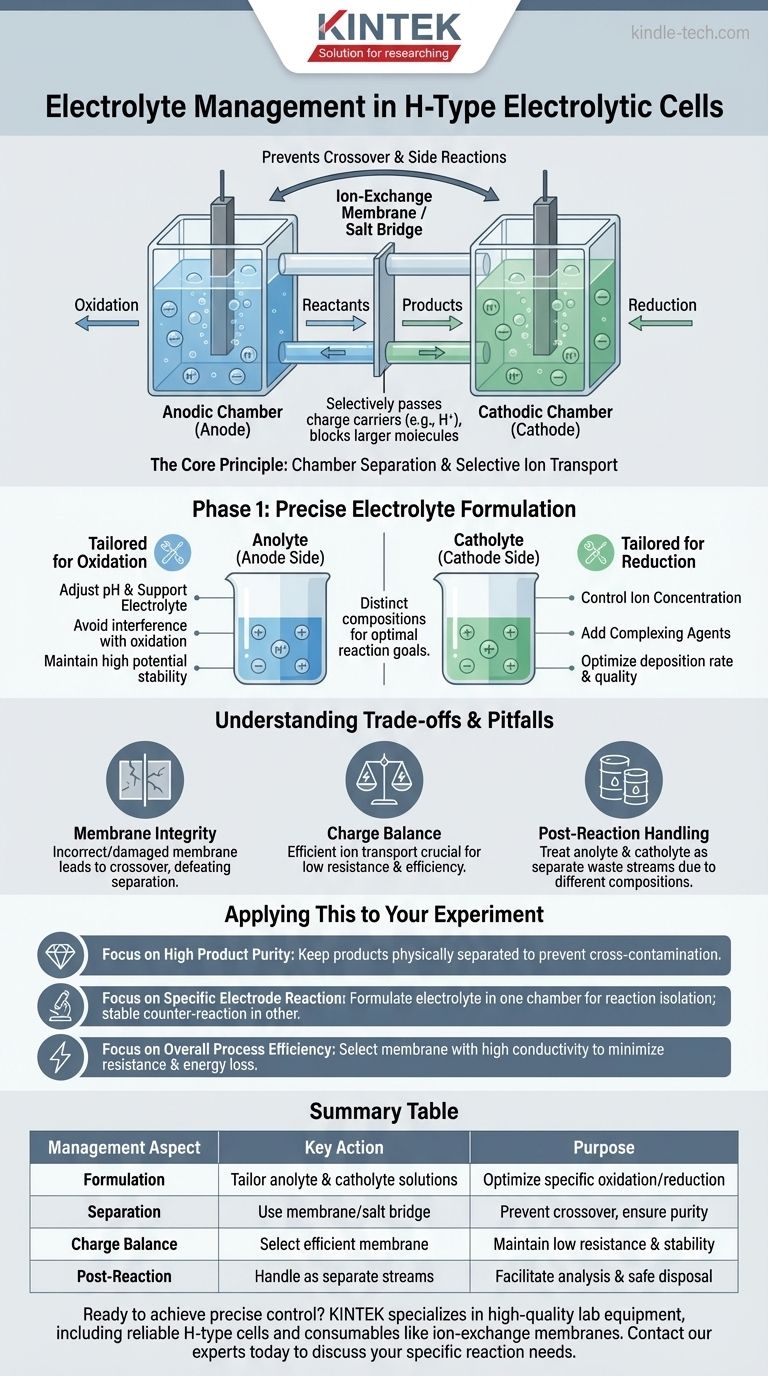
In an H-type electrolytic cell, the electrolyte is managed by formulating distinct solutions for the separate anodic and cathodic chambers. This allows for precise control over the components, concentration, and pH on each side of the cell, which is essential for optimizing specific reactions and preventing unwanted crossover of products or reactants.
The fundamental advantage of an H-type cell is its physical separation of the anode and cathode. Effective electrolyte management leverages this separation to create two unique chemical environments, allowing you to isolate and control specific electrochemical processes with high precision.

The Core Principle: Chamber Separation
An H-type cell, named for its characteristic shape, is a divided electrochemical cell. It consists of two separate compartments, one for the anode and one for the cathode, connected by an ion-exchange membrane or a salt bridge.
Why Separation is Critical
This divided structure is the key to its utility. It physically prevents the reactants, intermediates, and final products from the anode from mixing with those from the cathode.
This isolation is crucial for preventing side reactions, improving product purity, and allowing the use of different electrolyte compositions in each half-cell.
The Role of the Ion-Exchange Membrane
The connection between the chambers, typically a proton-exchange membrane (like Nafion) or a salt bridge, is not just a passive link.
It selectively allows certain ions (e.g., protons or other charge carriers) to pass through to maintain charge neutrality in the system while blocking the passage of larger molecules or specific reactants.
Phase 1: Precise Electrolyte Formulation
Effective management begins long before the experiment starts. The composition of the electrolyte in each chamber—the anolyte (anode side) and catholyte (cathode side)—is tailored to the specific reaction goal.
Tailoring the Catholyte for Reduction
The catholyte is designed to optimize the reduction reaction.
For instance, in an electroplating experiment, the catholyte's composition is paramount. Controlling the concentration of metal ions and adding specific complexing agents directly dictates the rate and quality of the metal deposition onto the cathode.
Tailoring the Anolyte for Oxidation
Simultaneously, the anolyte is formulated to support the desired oxidation reaction.
This might involve setting a different pH or using a different supporting electrolyte that won't interfere with the oxidation process or degrade at the high potential of the anode.
Understanding the Trade-offs and Pitfalls
While powerful, the H-type cell's design introduces complexities that must be managed carefully to ensure reliable results.
Membrane Selection and Integrity
The choice of membrane is critical. An incorrect or damaged membrane can lead to "crossover," where reactants or products leak from one chamber to the other, defeating the purpose of the cell.
Maintaining Charge Balance
The system relies on the efficient transport of ions through the membrane to balance the charge generated at the electrodes. Any impedance or blockage can increase cell resistance, reduce efficiency, and skew experimental data.
Post-Reaction Handling
Management extends to the end of the experiment. The products must be carefully removed for analysis or further processing.
Because the anolyte and catholyte can have different compositions and contain different byproducts, they must be treated as separate waste streams. Each must be disposed of according to environmental and safety regulations to prevent pollution.
Applying This to Your Experiment
Your approach to electrolyte management should be dictated by your primary experimental objective.
- If your primary focus is high product purity: Use the H-cell to keep your desired anodic and cathodic products physically separated, preventing cross-contamination and subsequent purification challenges.
- If your primary focus is studying a specific electrode reaction: Formulate the electrolyte in the chamber of interest to isolate that reaction, while using a simple, stable counter-reaction in the other chamber.
- If your primary focus is overall process efficiency: Select an ion-exchange membrane with high conductivity for your charge-carrying ion to minimize electrical resistance and energy loss.
Mastering the distinct management of the anolyte and catholyte is how you unlock the full potential of an H-type cell for precise electrochemical control.
Summary Table:
| Management Aspect | Key Action | Purpose |
|---|---|---|
| Formulation | Tailor distinct anolyte & catholyte solutions | Optimize specific oxidation/reduction reactions |
| Separation | Use ion-exchange membrane or salt bridge | Prevent reactant/product crossover, ensure purity |
| Charge Balance | Select membrane for efficient ion transport | Maintain low resistance and system stability |
| Post-Reaction | Handle anolyte and catholyte as separate streams | Facilitate analysis and ensure safe disposal |
Ready to achieve precise control over your electrochemical reactions? KINTEK specializes in high-quality lab equipment, including reliable H-type electrolytic cells and consumables like ion-exchange membranes. Our expertise helps laboratories optimize electrolyte management for superior product purity and experimental efficiency. Contact our experts today to discuss your specific reaction needs and find the perfect solution for your lab.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results



















