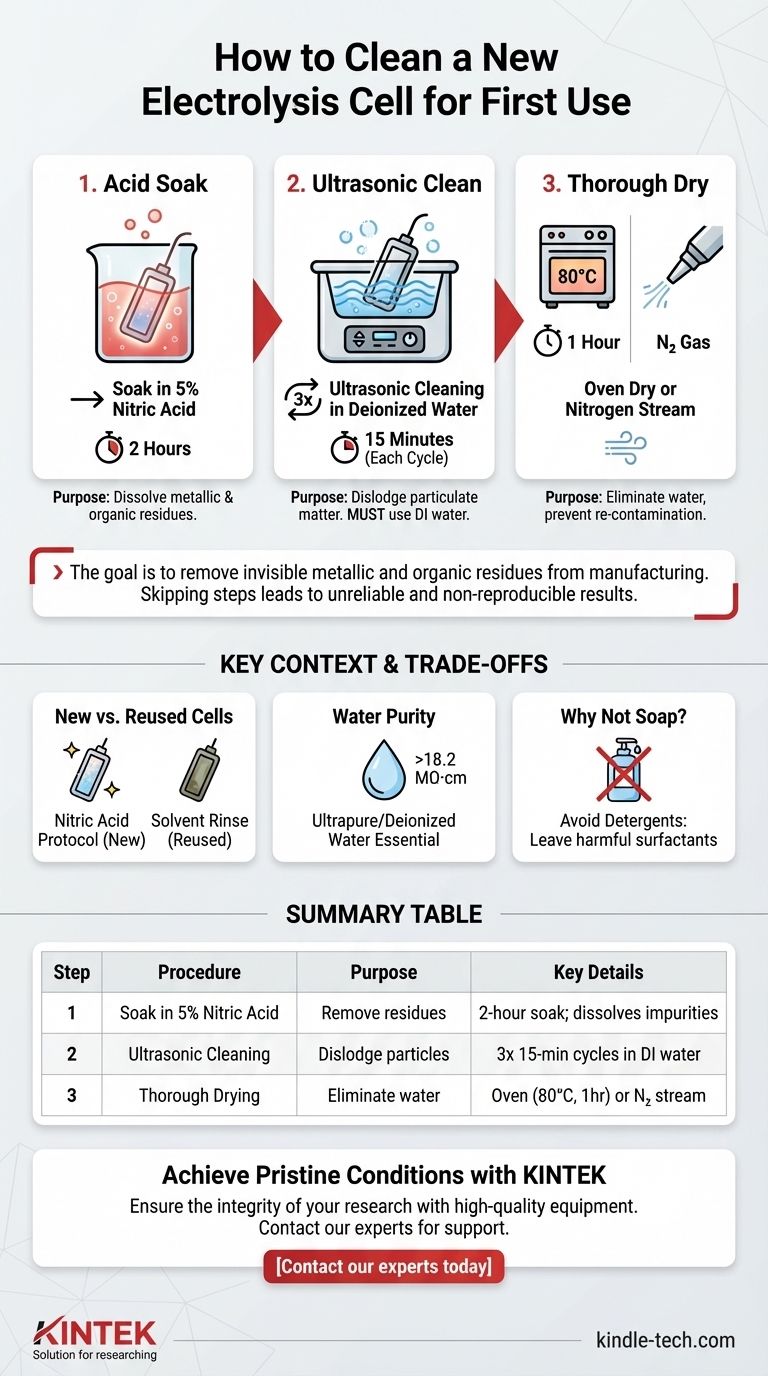
To properly clean a new electrolysis cell, it must be soaked in a 5% nitric acid solution for two hours. This is followed by three separate rounds of ultrasonic cleaning in deionized water, each lasting 15 minutes. Finally, the cell must be thoroughly dried, either in an oven at 80°C for one hour or by using a clean nitrogen gas stream.
The goal of this multi-step process is not just to clean the cell, but to remove invisible metallic and organic residues from manufacturing. Skipping these steps is one of the most common sources of experimental error, leading to unreliable and non-reproducible results.

The Rationale Behind Each Cleaning Step
Proper cell preparation is a prerequisite for accurate electrochemical work. Each step in the cleaning protocol is designed to eliminate a specific type of contaminant without introducing new ones.
Step 1: Nitric Acid Soaking
The initial soak in a 5% nitric acid solution is the most critical step for removing inorganic and metallic impurities left over from the glass or quartz manufacturing process.
As a strong oxidizing agent, nitric acid dissolves trace metals and oxidizes organic films that may be present on the surface, ensuring they can be easily rinsed away.
Step 2: Ultrasonic Cleaning with Deionized Water
Following the acid bath, ultrasonic cleaning provides mechanical agitation at a microscopic level. The high-frequency sound waves create tiny cavitation bubbles that dislodge any remaining particulate matter or stubborn residues from the cell's surface.
Using deionized (DI) or ultrapure water is non-negotiable. Tap water contains various ions (like chloride, calcium, and magnesium) that will contaminate the surface you just cleaned and interfere with your future experiments. Repeating the process three times ensures complete removal of the acid and all dislodged contaminants.
Step 3: Thorough Drying
The final step is to remove all residual water. Oven drying at 80°C is a reliable method, but it's crucial that the oven itself is clean.
Alternatively, drying with a gentle stream of high-purity nitrogen gas is often preferred. This method is faster, avoids thermal stress on the glass, and actively purges the cell with an inert atmosphere, preventing re-contamination from ambient air.
Understanding the Trade-offs and Context
While the protocol is straightforward, understanding the context is key to avoiding common pitfalls that can compromise your research.
New vs. Reused Cells: A Critical Distinction
The protocol described here is specifically for brand-new cells. The goal is to remove manufacturing residues.
Cleaning a reused cell targets different contaminants—namely, the reactants, products, and supporting electrolyte from your previous experiment. A typical procedure for a reused cell involves wiping with acetone to remove organics, followed by rinsing with ethanol and finally with ultrapure water.
Why Not Just Soap and Water?
Standard laboratory detergents or soaps should never be used. They are designed to leave behind a film of surfactants, which are electrochemically active and will ruin your measurements. The nitric acid protocol ensures no such organic film remains.
The Importance of Water Purity
The term "deionized" or "ultrapure" water refers to water with a resistivity greater than 18.2 MΩ·cm. This extremely high resistance indicates a near-total absence of free ions, making it an ideal and "blank" solvent for rinsing that won't introduce electrochemical interference.
Making the Right Choice for Your Experiment
Your adherence to this protocol should match the sensitivity of your work.
- If your primary focus is on trace analysis, catalysis, or sensor development: The full nitric acid and ultrasonic cleaning protocol is absolutely mandatory. There is no shortcut to achieving a pristine surface for this kind of sensitive work.
- If your primary focus is on a general chemistry demonstration or a less sensitive bulk electrolysis: While the full protocol remains best practice, a rigorous, multi-stage rinse with deionized water may be sufficient, but you must acknowledge the risk of introducing unknown variables.
- If you are reusing a cell from a previous experiment: Do not use the nitric acid protocol unless you need to strip everything off. Instead, use the appropriate solvent-based cleaning (e.g., acetone, ethanol, water) to remove your specific experimental residues.
Ultimately, starting with an impeccably clean cell is the first step toward generating data you can trust.
Summary Table:
| Step | Procedure | Purpose | Key Details |
|---|---|---|---|
| 1 | Soak in 5% Nitric Acid | Remove metallic/organic residues | 2-hour soak; dissolves trace impurities |
| 2 | Ultrasonic Cleaning | Dislodge particulate matter | 3x 15-min cycles in deionized water |
| 3 | Thorough Drying | Eliminate water and prevent contamination | Oven (80°C, 1hr) or high-purity nitrogen stream |
Achieve Pristine Experimental Conditions with KINTEK
Generating reliable, reproducible electrochemical data starts with a perfectly clean cell. The meticulous cleaning protocol outlined above is essential for removing invisible manufacturing residues that can skew your results. For researchers in catalysis, sensor development, and trace analysis, this level of preparation is non-negotiable.
KINTEK specializes in providing the high-quality lab equipment and consumables you need to execute these critical procedures with confidence. From durable electrolysis cells to high-purity reagents and reliable drying ovens, we supply the foundational tools for scientific excellence.
Ensure the integrity of your research from the very first step. Contact our experts today to discuss your specific laboratory requirements and how KINTEK can support your quest for accurate, trustworthy data.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results



















