The short answer is this: Steel must be heated in a slow, uniform, and highly controlled manner to a specific temperature, held there for a sufficient duration, and then cooled appropriately. This process is not a single action but a three-stage sequence—heating, soaking, and cooling—where the parameters for each stage are dictated entirely by the type of steel and the desired final properties. Rushing or mismanaging the heating phase is the most common cause of failed heat treatment.
The single most important principle of heating steel is control. Uncontrolled or non-uniform heating introduces thermal stress and causes incomplete metallurgical transformation, which are the primary sources of cracking, warping, and inconsistent material properties.

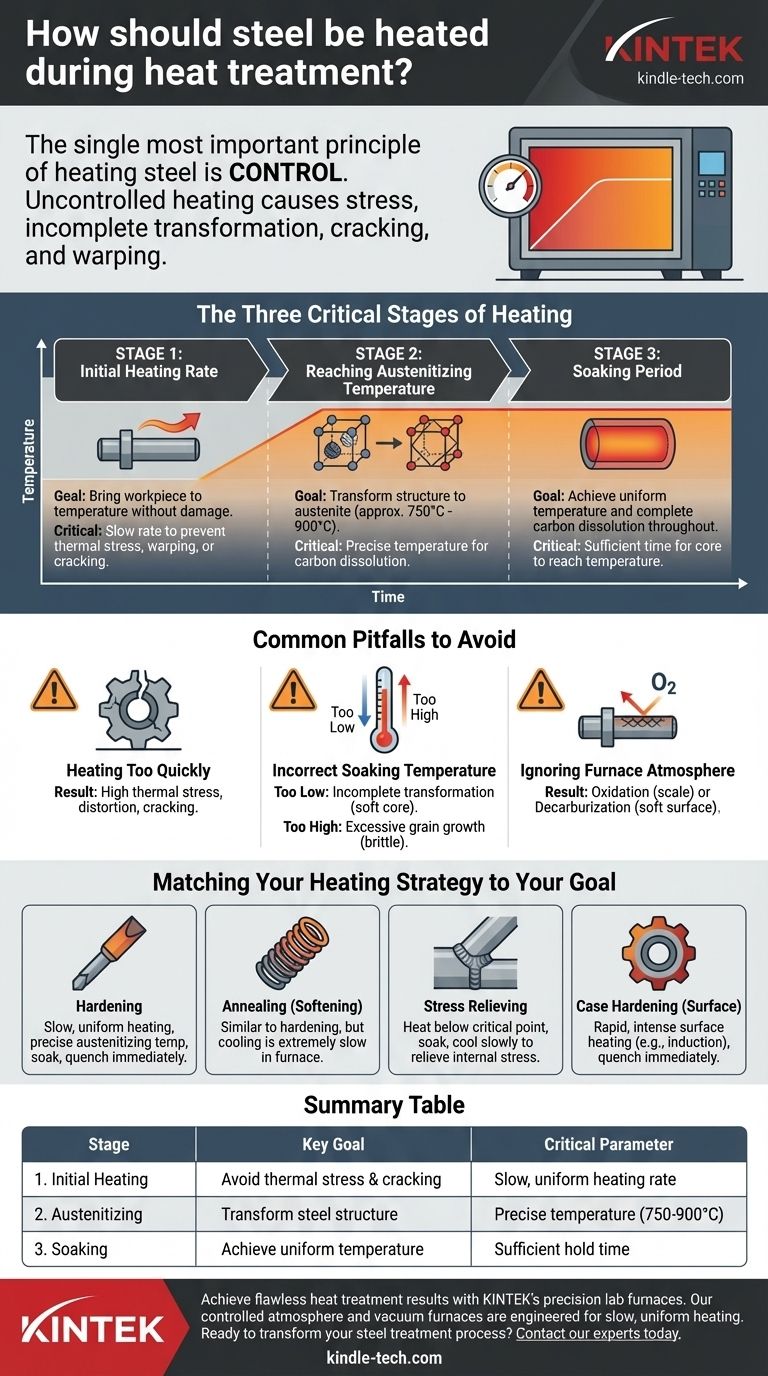
The Three Critical Stages of Heating
Successfully heating steel for treatment requires managing three distinct, sequential stages. Each one serves a critical purpose in preparing the steel's internal structure for the final transformation that occurs during cooling.
Stage 1: The Initial Heating Rate
The goal during the initial phase is to bring the workpiece up to the target temperature without causing damage. Heating steel causes it to expand, and if one part of a component heats up faster than another, this differential expansion creates internal stress.
For complex shapes, thick sections, or high-carbon steels, this thermal stress can easily exceed the material's strength, leading to warping or cracking before the steel ever reaches its transformation temperature. Therefore, the heating rate must be slow enough to allow the temperature to equalize throughout the part.
Stage 2: Reaching the Austenitizing Temperature
For hardening and normalizing, the steel must be heated above its upper critical temperature (typically between 750°C and 900°C or 1400°F and 1650°F, depending on the alloy). This is the austenitizing temperature.
At this point, the steel's crystal structure transforms from its room-temperature state (ferrite and pearlite) into a new, high-temperature structure called austenite. This new structure has the unique ability to dissolve carbon into a solid solution, which is the essential precondition for hardening during the subsequent quench.
Stage 3: The Soaking Period
Simply reaching the target temperature is not enough. The workpiece must be held at that temperature for a specific period, known as soaking.
The purpose of soaking is twofold. First, it ensures that the entire cross-section of the part, from the surface to the core, has reached a uniform temperature. Second, it provides the necessary time for the carbon and other alloying elements to dissolve completely and evenly throughout the austenite structure. Insufficient soak time is a primary cause of a hard surface but a soft, weak core.
Common Pitfalls to Avoid
The heating process is where most heat-treatment defects originate. Understanding these common mistakes is crucial for achieving predictable, high-quality results.
Pitfall 1: Heating Too Quickly
This is the most frequent error. The immediate consequence is high thermal stress, leading to distortion or cracking. This is especially dangerous for tool steels and parts with sharp corners or drastic changes in thickness.
Pitfall 2: Incorrect Soaking Temperature
Using the wrong temperature undermines the entire process.
- Undershooting (too low): The transformation to austenite will be incomplete. Carbon will not fully dissolve, and the steel will not achieve its maximum potential hardness after quenching.
- Overshooting (too high): This causes the crystalline grains within the austenite to grow excessively. Large grains result in a brittle and weak final product, even if it is hard.
Pitfall 3: Ignoring the Furnace Atmosphere
The environment in which the steel is heated matters immensely. Heating in the presence of oxygen (as in a standard air-fired furnace) can cause two major problems.
- Oxidation (Scale): A layer of iron oxide, or scale, forms on the surface. This alters the part's final dimensions and can interfere with the quenching process.
- Decarburization: Oxygen can react with and remove carbon from the steel's surface. A decarburized surface will not harden properly, resulting in a soft "skin" on the finished part. Using a vacuum furnace or introducing a protective atmosphere can prevent this.
Matching Your Heating Strategy to Your Goal
The right heating protocol depends entirely on what you are trying to achieve.
- If your primary focus is Hardening: Heat slowly and uniformly to the precise austenitizing temperature for your specific alloy, soak long enough for the core to reach temperature, and then proceed immediately to quenching.
- If your primary focus is Annealing (Softening): The heating process is similar to hardening, but the cooling that follows must be extremely slow, often allowing the part to cool down with the furnace itself.
- If your primary focus is Stress Relieving: Heat to a temperature well below the critical transformation point, hold for uniformity, and then cool slowly. The goal is to relieve internal stress without altering the core hardness.
- If your primary focus is Case Hardening (Surface Hardening): Use methods like induction or flame heating that apply intense heat very rapidly and only to the surface, leaving the core unaffected before quenching.
Ultimately, mastering the controlled application of heat is the foundation of predictable and successful steel heat treatment.
Summary Table:
| Stage | Key Goal | Critical Parameter |
|---|---|---|
| 1. Initial Heating | Avoid thermal stress & cracking | Slow, uniform heating rate |
| 2. Austenitizing | Transform steel structure | Precise temperature (750-900°C) |
| 3. Soaking | Achieve uniform temperature & carbon dissolution | Sufficient hold time at temperature |
Achieve flawless heat treatment results with KINTEK's precision lab furnaces.
Our controlled atmosphere and vacuum furnaces are engineered to provide the slow, uniform heating essential for preventing warping, cracking, and decarburization. Whether your goal is hardening, annealing, or stress relieving, KINTEK equipment delivers the precision and reliability your laboratory demands.
Ready to transform your steel treatment process? Contact our experts today to find the perfect furnace solution for your specific alloy and application.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the maximum temperature of a muffle furnace? Find the Right Heat for Your Application
- How long is the calcination process? Optimize Your Process Time for Maximum Efficiency
- What is the use of muffle furnace in soil? Analyze Soil Composition with High-Temperature Precision
- How to use a muffle furnace in a laboratory? A Step-by-Step Guide to Safe, Precise Thermal Processing
- How to maintain a muffle furnace? Ensure Long-Term Reliability and Safety



















