Proper electrode maintenance is essential for ensuring the accuracy, repeatability, and efficiency of any electrochemical process. The core practice involves a cycle of careful inspection for damage, thorough cleaning to remove contaminants and reaction byproducts, and correct storage to prevent degradation from oxidation or moisture. These steps preserve the electrode's active surface, which is critical for its performance.
The goal of electrode maintenance extends beyond simple cleanliness. It is fundamentally about preserving the integrity of the electrode's active surface, as any contamination, oxidation, or physical damage directly compromises experimental results and reduces the lifespan of the cell.

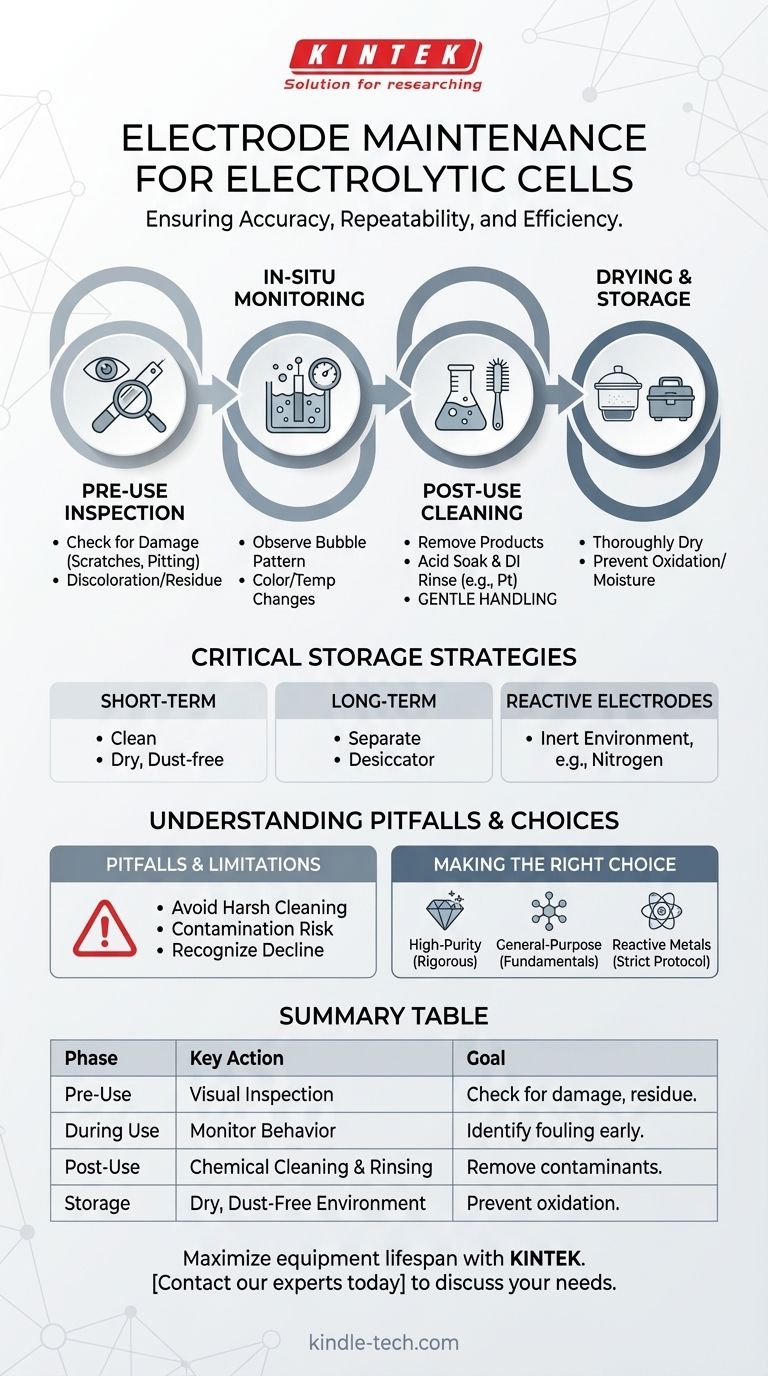
The Maintenance Lifecycle: Before, During, and After Use
A systematic approach to maintenance, integrated into your experimental workflow, is the most effective way to guarantee electrode longevity and performance.
Pre-Use Inspection: Your First Line of Defense
Before every use, visually inspect the electrode surfaces. Look for any signs of physical damage like scratches, pitting, or deformation. Also check for discoloration, residue from a previous experiment, or signs of corrosion that could interfere with your reaction.
In-Situ Monitoring: Catching Problems Live
During an experiment, pay attention to the cell's behavior. Observe the pattern of bubble formation on the electrodes, any unexpected color changes in the electrolyte, or significant temperature fluctuations. These can be early indicators of electrode fouling, side reactions, or other issues that need to be addressed.
Post-Use Cleaning: Resetting the Surface
Immediately after use, clean the electrodes to remove any deposited reaction products or surface films. The exact method depends on the electrode material and the electrolyte used.
For noble metal electrodes like platinum, a common procedure is to soak them in a dilute acid (such as 1M nitric acid) and then rinse them thoroughly with deionized water. The goal is to chemically remove adsorbates without damaging the electrode itself.
Drying and Handling: Preventing Accidental Damage
After cleaning, the electrodes and all cell components must be dried thoroughly, as residual moisture can lead to corrosion or contamination.
Always handle electrodes and glass cell components gently. The delicate surface of an electrode can be easily scratched, altering its surface area and electrochemical properties.
Critical Storage Strategies for Longevity
How you store your electrodes between experiments is as important as how you clean them. Improper storage is a primary cause of performance degradation.
Short-Term Storage
For storage between frequent uses, ensuring the electrodes are clean and completely dry is sufficient. Store them in a dust-free, dry environment where they will not be subject to physical shock.
Long-Term Storage
If the cell will not be used for an extended period, it is best practice to pour out the electrolyte for separate, sealed storage. The dried electrodes and cell components should be stored in a non-humid cabinet or a desiccator.
The Challenge of Reactive Electrodes
Electrodes made of metals prone to oxidation (e.g., copper, zinc, iron) require special care. Prolonged exposure to air will cause an insulating oxide layer to form on the surface, rendering the electrode passive.
To prevent this, store these electrodes in a dry, oxygen-free environment (like a nitrogen-filled glovebox) or immerse them in a protective, non-reactive solution.
Understanding the Pitfalls and Limitations
Effective maintenance requires judgment. An overly aggressive approach can be as harmful as neglect.
When Cleaning Becomes Damaging
Avoid harsh physical cleaning methods like scrubbing or scraping, which can permanently damage the electrode surface. Similarly, using overly aggressive chemicals can etch or corrode the electrode, fundamentally changing its behavior. Always use the mildest cleaning agent that is effective.
The Hidden Risk of Contamination
Even trace amounts of contamination from improper cleaning or storage can have a major impact. Contaminants can passivate the electrode surface, blocking reactions, or act as an unintended catalyst for side reactions, compromising the purity of your results.
Recognizing Performance Decline
All electrodes have a finite lifespan. If you observe a consistent decline in performance (e.g., higher overpotential, lower current efficiency) despite following a proper maintenance routine, the electrode surface may be irrevocably damaged or fouled. At this point, it requires professional treatment (like polishing and re-plating) or replacement.
Making the Right Choice for Your Goal
Your maintenance protocol should align with your experimental needs. A "one-size-fits-all" approach is rarely optimal.
- If your primary focus is high-purity synthesis or trace analysis: Your protocol must be rigorous. Use dedicated electrodes for specific reaction types to prevent cross-contamination and employ stringent acid-washing and rinsing steps.
- If your primary focus is general-purpose electrolysis or teaching: Prioritize the fundamentals. Consistently inspecting, rinsing with deionized water, and ensuring proper drying will maximize equipment lifespan and provide reliable results.
- If you are working with non-noble or reactive metal electrodes: Your main concern is oxidation. Implement a strict storage protocol using either an inert atmosphere or a protective solution to prevent surface passivation between uses.
By treating electrode care as an integral part of the scientific process, you transform your components from consumables into reliable, long-lasting instruments.
Summary Table:
| Maintenance Phase | Key Action | Goal |
|---|---|---|
| Pre-Use | Visual Inspection | Check for damage, discoloration, or residue. |
| During Use | Monitor Behavior | Identify fouling or side reactions early. |
| Post-Use | Chemical Cleaning & Rinsing | Remove reaction products and contaminants. |
| Storage | Dry, Dust-Free Environment | Prevent oxidation and physical damage. |
Maximize the performance and lifespan of your lab's electrochemical equipment. Proper electrode maintenance is critical for accurate results. KINTEK specializes in high-quality lab equipment and consumables, including electrochemical cells and accessories, to support your research and teaching needs.
Contact our experts today to discuss your specific requirements and ensure your experiments run with precision and reliability.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What is the role of titanium wire in seawater SMFCs? Ensure Stability & Protect Microbial Life
- What are the materials used for the components of the PTFE electrode stand? A Guide to Chemical Resistance & Stability
- What is the typical lifespan of a platinum disk electrode? Maximize Performance with Proper Care
- What are the technical advantages of using a spiral platinum wire? Optimize Your Electrochemical Precision
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- How should a titanium electrode be regularly maintained and cleaned? Protect Your Investment and Maximize Performance
- Why is the selection of a high-quality reference electrode critical in the electrochemical synthesis? | KINTEK
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained



















