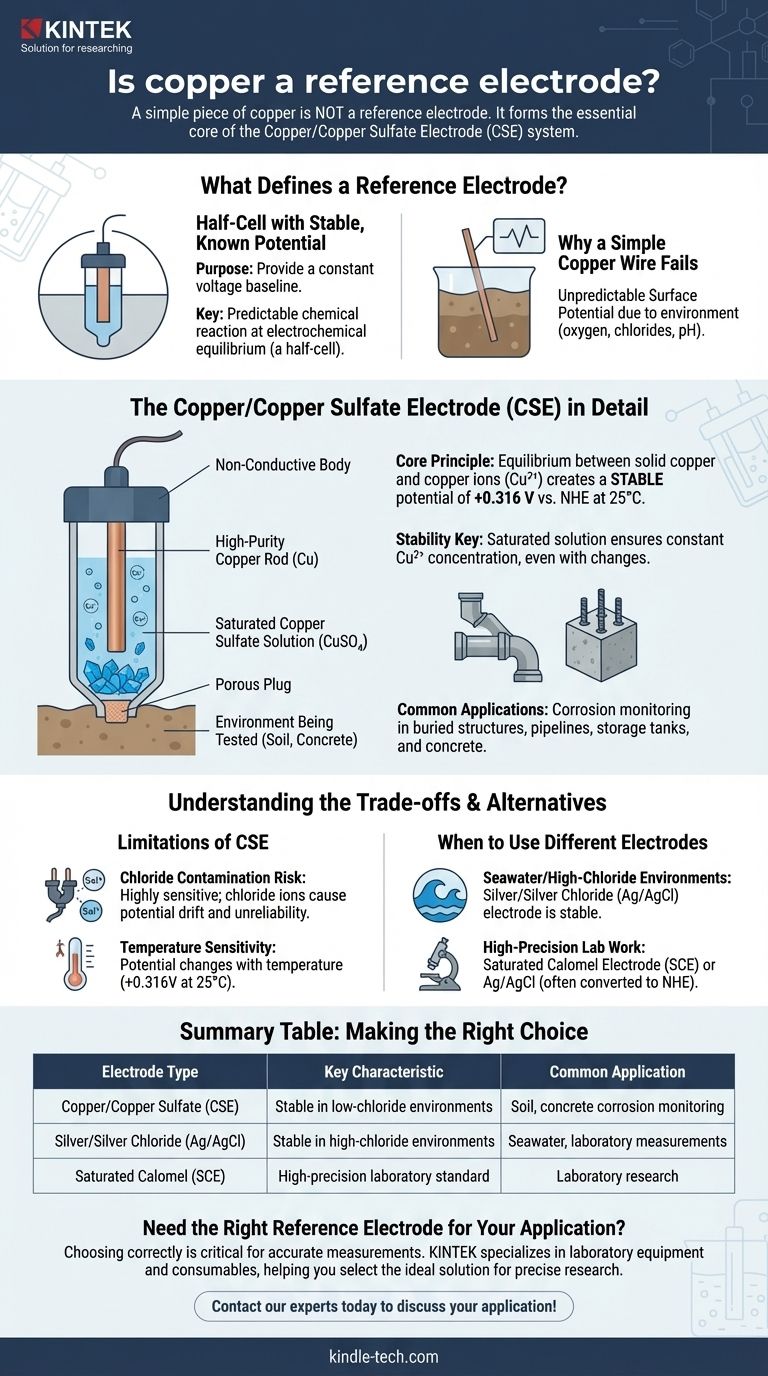
While a simple piece of copper is not a reference electrode, it forms the essential core of a very common one: the Copper/Copper Sulfate Electrode (CSE). For copper to function as a reference, it must be immersed in a specific, saturated solution of its own salt (copper sulfate). This complete system, not the metal alone, creates the stable and predictable electrical potential required for a reliable reference.
The critical distinction is that a reference electrode isn't just a piece of metal; it's a complete electrochemical half-cell with a stable, known potential. Copper only achieves this stability when it is part of a system, most commonly as a Copper/Copper Sulfate Electrode (CSE).

What Defines a Reference Electrode?
To understand why a simple copper wire isn't sufficient, we must first define the job of a reference electrode. Its sole purpose is to provide a constant voltage baseline against which other, unknown potentials can be measured.
The Principle of a Stable Potential
A reference electrode must have a potential that does not change, even when small currents pass through it or when the surrounding environment fluctuates. It is the stable "zero point" on your electrochemical ruler.
The Role of Electrochemical Equilibrium
This stability comes from a predictable chemical reaction at equilibrium. A true reference electrode is a half-cell where a metal is in contact with a fixed concentration of its own ions in a solution.
For the CSE, this equilibrium is between solid copper (Cu) and copper ions (Cu²⁺) in the solution. This creates a known potential of +0.316 Volts relative to the Normal Hydrogen Electrode (NHE) at 25°C.
Why a Simple Copper Wire Fails
If you place a random piece of copper into an environment like soil or water, its surface potential will be unpredictable. It will vary wildly based on the local concentration of oxygen, chlorides, pH, and other ions, making it useless as a stable reference point.
The Copper/Copper Sulfate Electrode (CSE) in Detail
The CSE is the practical application of this principle and is an industry workhorse, particularly in corrosion monitoring.
Construction and Components
A typical CSE consists of a high-purity copper rod submerged in a saturated solution of copper sulfate (CuSO₄). This entire assembly is housed in a non-conductive body with a porous plug at the tip, which allows electrical contact with the environment being tested (e.g., soil or concrete).
How It Maintains Stability
The key to its stability is the saturated solution. As long as there are undissolved copper sulfate crystals present, the concentration of copper ions (Cu²⁺) in the solution remains constant, even with slight changes in temperature or water evaporation. This constant ion concentration is what locks in the stable potential.
Common Applications
The CSE is the standard reference electrode for applications involving buried structures. It is used extensively to measure the potential of steel pipelines, storage tanks, and the reinforcing steel (rebar) in concrete to assess corrosion and the effectiveness of cathodic protection systems.
Understanding the Trade-offs
While robust, the CSE is not universally ideal. Understanding its limitations is crucial for accurate measurements.
The Risk of Contamination
The CSE is highly sensitive to chloride contamination. If chloride ions from the soil or concrete leak through the porous plug, they can react with the copper rod and the copper sulfate solution, causing the electrode's potential to drift and become unreliable.
Temperature Sensitivity
The standard +0.316 V potential is specified at 25°C (77°F). The electrode's potential changes with temperature, a factor that must be corrected for if high precision is required across a wide range of conditions.
When to Use a Different Electrode
Because of its chloride sensitivity, the CSE is generally unsuitable for use in seawater or other high-chloride environments. In these cases, a Silver/Silver Chloride (Ag/AgCl) electrode is the preferred choice, as its chemistry is inherently stable in the presence of chlorides.
Making the Right Choice for Your Measurement
Choosing the correct reference electrode is fundamental to getting meaningful data. Your decision should be guided by the chemical environment you are measuring.
- If your primary focus is measuring potentials in soil or concrete: The Copper/Copper Sulfate Electrode (CSE) is the industry standard due to its robustness and stability in these low-chloride environments.
- If your primary focus is working in seawater, estuaries, or other high-chloride solutions: A Silver/Silver Chloride (Ag/AgCl) electrode is the correct choice to ensure accuracy and avoid contamination-induced potential drift.
- If your primary focus is high-precision laboratory work: A Saturated Calomel Electrode (SCE) or Ag/AgCl is often used, and all results are typically converted and reported relative to the theoretical Normal Hydrogen Electrode (NHE) for universal comparison.
Understanding that a reference electrode is a complete electrochemical system, not just a material, is the key to making reliable measurements.
Summary Table:
| Electrode Type | Key Characteristic | Common Application |
|---|---|---|
| Copper/Copper Sulfate (CSE) | Stable potential in low-chloride environments | Soil, concrete corrosion monitoring |
| Silver/Silver Chloride (Ag/AgCl) | Stable in high-chloride environments | Seawater, laboratory measurements |
| Saturated Calomel (SCE) | High-precision laboratory standard | Laboratory research |
Need the Right Reference Electrode for Your Application?
Choosing the correct reference electrode is critical for accurate and reliable electrochemical measurements. Whether you're monitoring corrosion in soil or conducting high-precision lab analysis, the wrong choice can lead to significant data errors.
KINTEK specializes in laboratory equipment and consumables. We can help you select the ideal reference electrode and associated lab equipment for your specific needs, ensuring your measurements are precise and your research is sound.
Contact our experts today to discuss your application and find the perfect solution!
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Glassy Carbon Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What are the advantages and disadvantages of the ceramic core type copper sulfate reference electrode?
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements
- What is the expected lifespan of a copper sulfate reference electrode? Maximize Longevity with Proper Maintenance
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained
- What is the operating principle of a copper sulfate reference electrode? Reliable Potential Measurement Explained



















