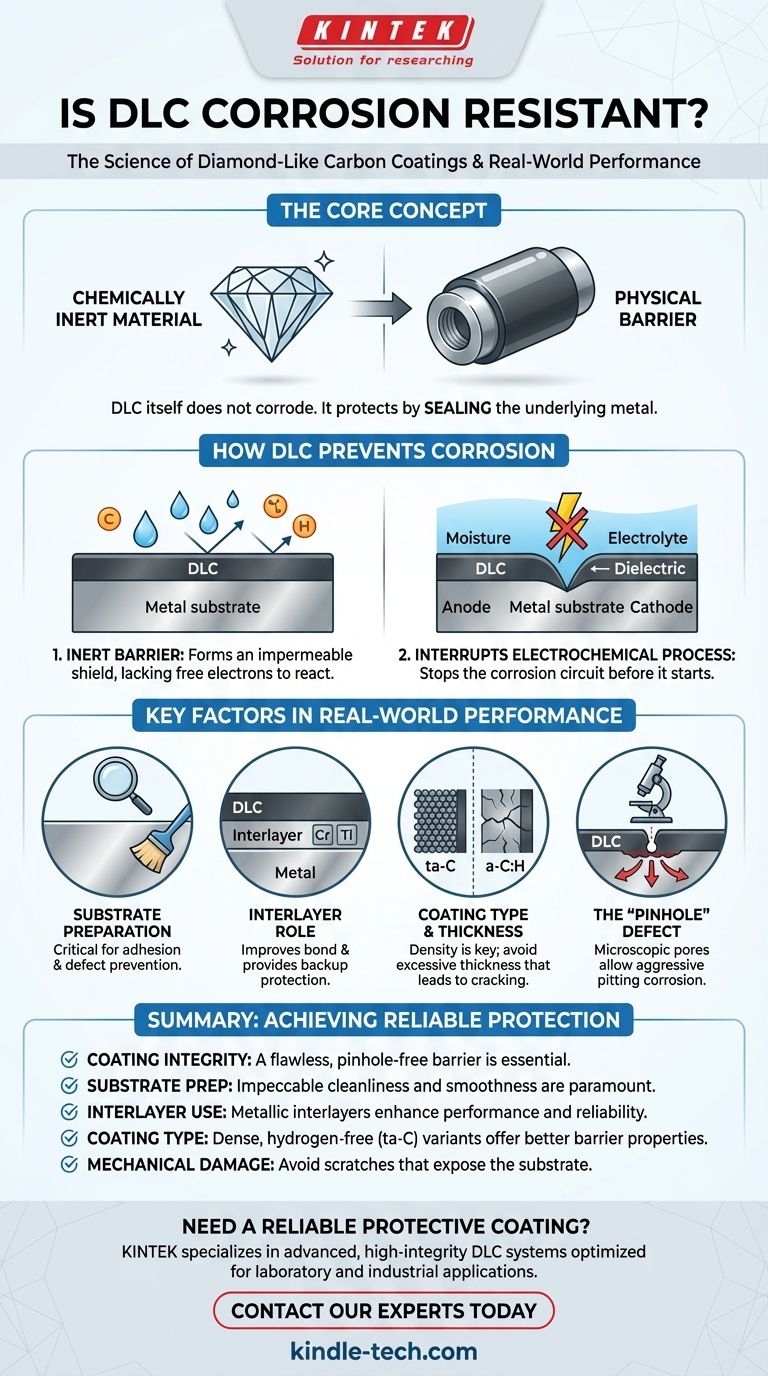
In principle, yes. Diamond-Like Carbon (DLC) coatings are highly effective at resisting corrosion because the material itself is chemically inert. However, its real-world performance is not about the material alone; it depends entirely on the quality of the coating's application and its integrity as a flawless physical barrier.
The core concept to understand is that DLC does not corrode, but it protects by sealing the underlying metal from the environment. Its effectiveness hinges on being a perfect, unbroken seal; any defect, like a microscopic pinhole, can compromise the protection and sometimes even accelerate localized corrosion.

How DLC Prevents Corrosion
A DLC coating functions less like a corrosion-resistant alloy and more like a high-performance raincoat for the substrate material. Its protective mechanism is based on creating an impermeable and inert barrier.
The Principle of the Inert Barrier
Fundamentally, DLC is a form of amorphous carbon with a molecular structure that lacks the free electrons and chemical reactivity of metals. This makes it chemically inert, much like glass or a noble metal.
It does not react with most common corrosive agents, including acids, alkalis, saltwater, and organic solvents. The coating itself simply will not degrade or rust.
Interrupting the Electrochemical Process
Corrosion is an electrochemical process that requires an anode, a cathode, and an electrolyte to create a circuit. A metal substrate (like steel) acts as the anode/cathode, and moisture acts as the electrolyte.
DLC, being a dielectric (an electrical insulator), physically separates the metal from the electrolyte. This separation stops the electrochemical reaction from ever starting.
The Importance of a Dense Structure
The effectiveness of this barrier depends entirely on the coating's physical structure. A high-quality DLC film is extremely dense and non-porous.
This density is what prevents molecules of water, oxygen, or salts from penetrating the coating and reaching the reactive metal substrate beneath it.
Key Factors in Real-World Performance
Not all DLC coatings are created equal. The difference between a coating that provides robust corrosion protection and one that fails prematurely lies in the details of the coating system.
Substrate Preparation is Critical
The substrate surface must be impeccably clean and smooth before coating. Any microscopic contamination, oxide layer, or surface roughness can lead to poor adhesion or the formation of defects in the final DLC film.
These defects become the weak points where corrosion will initiate.
The Role of an Interlayer
Most high-performance DLC applications are not just a single layer. They often include a metallic adhesion layer or interlayer (like chromium, titanium, or CrN) between the substrate and the DLC topcoat.
This interlayer dramatically improves the bond between the DLC and the substrate. Furthermore, if a defect does form in the DLC, this more corrosion-resistant interlayer can provide a secondary layer of protection.
Coating Type and Thickness
Different methods of depositing DLC produce films with varying densities, internal stresses, and hydrogen content. For example, hydrogen-free (ta-C) DLC is typically denser and provides a better barrier than hydrogenated (a-C:H) variants.
However, simply making the coating thicker is not always better. Thicker films can build up high internal stress, making them more brittle and prone to cracking, which would be catastrophic for corrosion protection.
Understanding the Trade-offs and Limitations
While powerful, DLC is not a universal solution. Understanding its potential failure modes is essential for successful application.
The "Pinhole" Defect
The most significant vulnerability of any barrier coating is a pinhole. This is a microscopic defect or pore that penetrates the entire coating thickness.
Even one pinhole creates a direct path for the corrosive environment to attack the substrate. This can lead to highly aggressive pitting corrosion, as the small exposed area of the substrate becomes an anode to the very large cathodic area of the inert DLC coating.
Susceptibility to Physical Damage
DLC is extremely hard, but it is also a very thin and relatively brittle film. A deep scratch, impact, or gouge that fully penetrates the coating will expose the substrate.
Once the substrate is exposed, corrosion will begin in that localized area, and it can then spread underneath the coating, causing it to flake off (delaminate).
Making the Right Choice for Your Application
Selecting the right DLC system requires defining your primary goal and the severity of the operating environment.
- If your primary focus is protection against mild humidity or occasional chemical splash: A standard, well-applied DLC coating is often an excellent and sufficient barrier.
- If your primary focus is resistance to aggressive environments like saltwater or constant chemical exposure: You must specify a multi-layer system with a corrosion-resistant interlayer and a high-integrity, virtually pinhole-free DLC topcoat.
- If your component is subject to high mechanical stress or impact: Consider a more ductile DLC formulation or a duplex treatment where the substrate is hardened (e.g., nitriding) before coating to provide better support for the hard film.
Ultimately, viewing DLC not as a material but as an engineered coating system is the key to achieving reliable corrosion protection.
Summary Table:
| Factor | Impact on Corrosion Resistance |
|---|---|
| Coating Integrity | A flawless, pinhole-free barrier is essential; any defect can compromise protection. |
| Substrate Preparation | Impeccable surface cleaning and smoothness are critical for strong adhesion and defect prevention. |
| Interlayer Use | A metallic interlayer (e.g., Cr, Ti) improves adhesion and provides secondary corrosion protection. |
| Coating Type/Thickness | Denser, hydrogen-free (ta-C) DLC offers better barrier properties; thickness must be optimized to avoid brittleness. |
| Mechanical Damage | Scratches or impacts that penetrate the coating will expose the substrate and initiate corrosion. |
Need a Reliable Protective Coating for Your Components?
DLC's effectiveness hinges on precise application and a deep understanding of material science. KINTEK specializes in advanced coating solutions, including high-integrity DLC systems, tailored for laboratory, industrial, and R&D applications. We ensure your components are protected against corrosive environments with coatings that are optimized for adhesion, density, and durability.
Contact our experts today to discuss how our DLC coatings can provide the impermeable barrier your critical components require.
Visual Guide
Related Products
- Custom CVD Diamond Coating for Lab Applications
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Electrolytic Electrochemical Cell for Coating Evaluation
- High-Purity Titanium Foil and Sheet for Industrial Applications
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- How does PECVD process work? Achieve Low-Temperature, High-Quality Thin Films
- What is thermal plasma chemical vapor deposition? Achieve Superior Coatings for Demanding Applications
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What are the process advantages of utilizing PECVD to produce graphene nanowalls from natural essential oils?
- What are plasma deposition reactors how and why are these used? Unlock Precision Thin-Film Deposition
- How long does DLC coating last? Unlock Extreme Durability for Your Components
- How strong is DLC coating? Discover the Ultimate Shield for Wear and Friction
- Can PECVD deposit metals? Discover the superior methods for pure metal thin films











