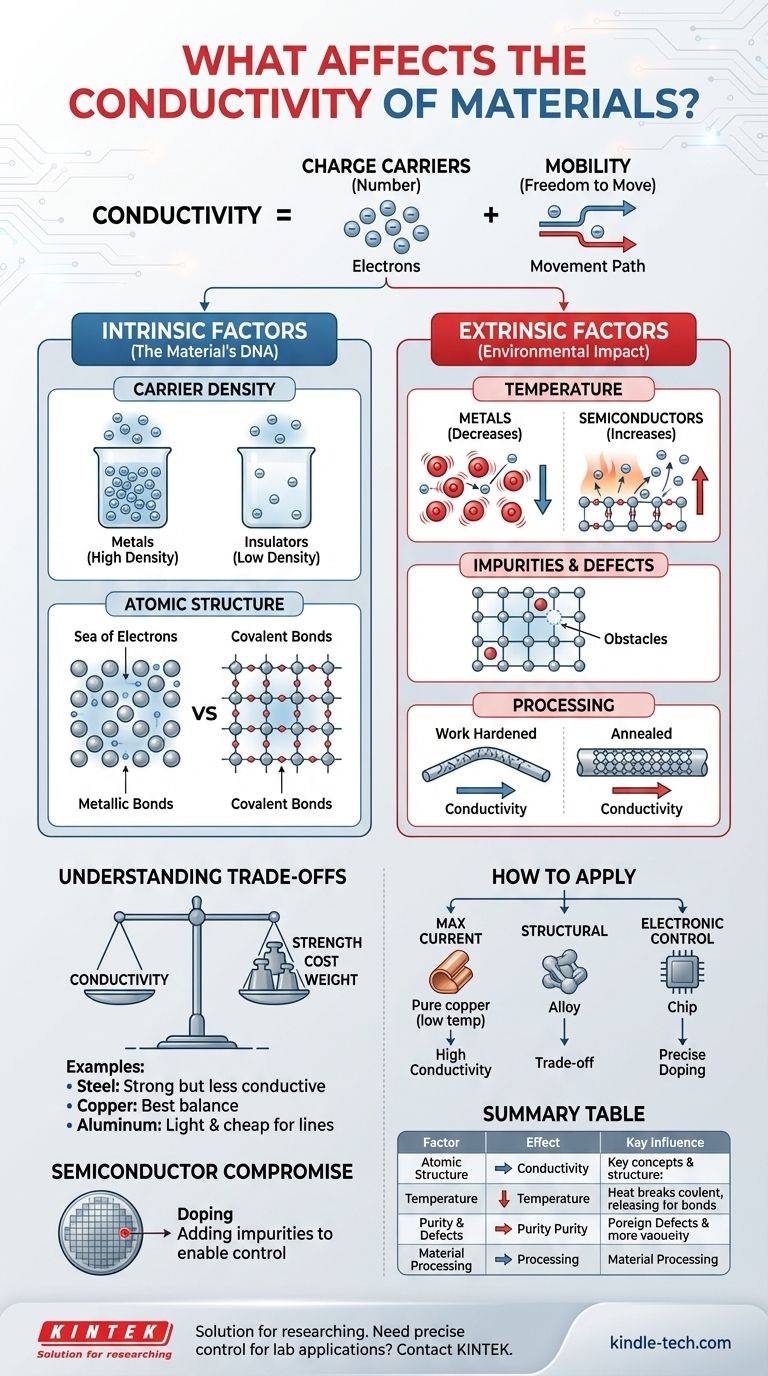
At its core, a material's electrical conductivity is determined by two fundamental properties: the number of mobile charge carriers it contains and how freely those carriers can move. Factors that influence these two properties—such as the material's atomic structure, temperature, and purity—are what ultimately control how well it conducts electricity.
A material’s conductivity is not a fixed number. It is a dynamic property reflecting the constant tension between the availability of charge carriers and the obstacles that impede their flow through the material's atomic lattice.
The Foundation: Charge Carriers and Mobility
To understand conductivity, we must first understand the two components that create it. Everything else is a factor that influences one or both of these components.
The Key Players: Charge Carriers
A charge carrier is a mobile particle that possesses an electric charge. In most common materials, the primary charge carrier is the electron.
Metals have a vast "sea" of free electrons that are not bound to any single atom, making them readily available to move and carry a current. Insulators, by contrast, have their electrons tightly bound, leaving very few available.
The Freedom to Move: Mobility
Mobility describes how easily these charge carriers can move through the material when an electric field (a voltage) is applied.
High mobility means carriers can accelerate quickly and travel far before being scattered or deflected. Low mobility means they are constantly colliding with obstacles and their net movement is hindered.
Intrinsic Factors: The Material's DNA
These factors are inherent to the material's chemical composition and atomic structure. They set the baseline for its potential conductivity.
Carrier Density
This is simply the concentration of available charge carriers. A material with a higher density of free carriers has a greater potential for high conductivity.
This is the primary reason metals are excellent conductors. Their atomic structure inherently produces a very high density of free electrons, often one or two per atom.
Atomic Structure and Bonding
The type of chemical bond holding the atoms together is critical. Metallic bonds create the delocalized "sea of electrons" perfect for conduction.
In contrast, covalent bonds, common in insulators and semiconductors, lock electrons between specific atoms. It takes a significant amount of energy to break these electrons free to act as charge carriers.
Extrinsic Factors: The Environmental Impact
These factors are not part of the material's ideal composition but arise from its environment, processing, or imperfections. They primarily affect carrier mobility by creating obstacles.
The Critical Role of Temperature
Temperature's effect on conductivity is one of the most important distinguishing factors between material types.
For metals, increasing temperature causes atoms to vibrate more intensely. These vibrations act as "speed bumps," scattering the free electrons more frequently. This reduces mobility and therefore decreases conductivity.
For semiconductors, increasing temperature provides the energy needed to break covalent bonds, releasing more electrons to become charge carriers. This increases carrier density so dramatically that it outweighs the minor loss in mobility, causing conductivity to increase.
Impurities and Crystal Defects
A perfect, repeating crystal lattice provides the clearest path for electrons. Any disruption to this pattern acts as a scattering site that reduces mobility.
Impurities (foreign atoms) and crystal defects (like vacancies or dislocations) distort the lattice, hindering electron flow and lowering conductivity. This is why highly pure copper is a better conductor than copper alloys like brass.
Material Processing
Mechanical processes like bending, rolling, or drawing a metal can introduce a high density of defects, a phenomenon known as work hardening. While this increases mechanical strength, it also reduces electrical conductivity.
Heating a metal (annealing) can repair many of these defects, restoring a more ordered crystal structure and increasing its conductivity.
Understanding the Trade-offs
Selecting a material is rarely about maximizing conductivity alone. It is almost always a compromise between competing properties.
Conductivity vs. Mechanical Strength
Adding other elements to a pure metal to form an alloy almost always increases strength and hardness but significantly degrades conductivity. The alloying atoms act as powerful scattering centers for electrons.
For example, steel is vastly stronger than pure iron, but it is also a much poorer electrical conductor.
Conductivity vs. Cost and Weight
Silver is the most conductive metal, but its cost makes it impractical for most applications. Copper offers the best balance of high conductivity and moderate cost, making it the standard for electrical wiring.
Aluminum is another common choice, especially for long-distance power lines. While only about 60% as conductive as copper, it is much lighter and cheaper, making it a better choice for that specific engineering problem.
The Semiconductor Compromise
Semiconductors like silicon are intrinsically poor conductors. However, their defining feature is that their conductivity can be precisely and dramatically increased by introducing specific impurities, a process called doping. This allows engineers to create materials with tailored electrical properties, which is the foundation of all modern electronics.
How to Apply This Knowledge
Choosing or evaluating a material requires balancing these factors against your specific goal.
- If your primary focus is maximum current transfer: Prioritize pure metals like annealed, oxygen-free copper and, if possible, operate at lower temperatures to minimize resistance.
- If your primary focus is structural integrity with moderate conductivity: Consider alloys like aluminum alloys (for aerospace) or bronze (for connectors), accepting the reduction in conductivity as a necessary trade-off for mechanical performance.
- If your primary focus is precise electronic control: Use semiconductors, where you intentionally manipulate carrier density through doping to create devices with specific functions.
Understanding these principles empowers you to look beyond a material's datasheet and predict its real-world electrical performance.
Summary Table:
| Factor | Effect on Conductivity | Key Influence |
|---|---|---|
| Atomic Structure | Defines baseline potential | Carrier density & mobility |
| Temperature | Decreases in metals, increases in semiconductors | Carrier scattering & activation |
| Purity & Defects | Reduces conductivity | Increases electron scattering |
| Material Processing | Can increase or decrease conductivity | Alters crystal lattice order |
Need precise control over material conductivity for your lab applications? KINTEK specializes in providing high-quality lab equipment and consumables that help you analyze and manipulate material properties with accuracy. Whether you're working with pure metals, alloys, or semiconductors, our tools support your research and development goals. Contact us today to discuss how we can enhance your laboratory's capabilities!
Visual Guide
Related Products
- E Beam Crucibles Electron Gun Beam Crucible for Evaporation
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Customizable XRD Sample Holders for Diverse Research Applications
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Customizable PTFE Wafer Carriers for Semiconductor and Lab Applications
People Also Ask
- What is sputtering technology? A Guide to Precision Thin Film Deposition
- What is the container that holds the metal source material called in e-beam evaporation? Ensure Purity and Quality in Your Thin-Film Deposition
- What is sputter coating used for? Achieve Superior Thin Films for Electronics, Optics, and Tools
- What are the effects of magnetron sputtering? Achieve High-Quality, Durable Thin Films for Your Lab
- What is a magnetron sputtering? A Guide to High-Quality Thin-Film Deposition