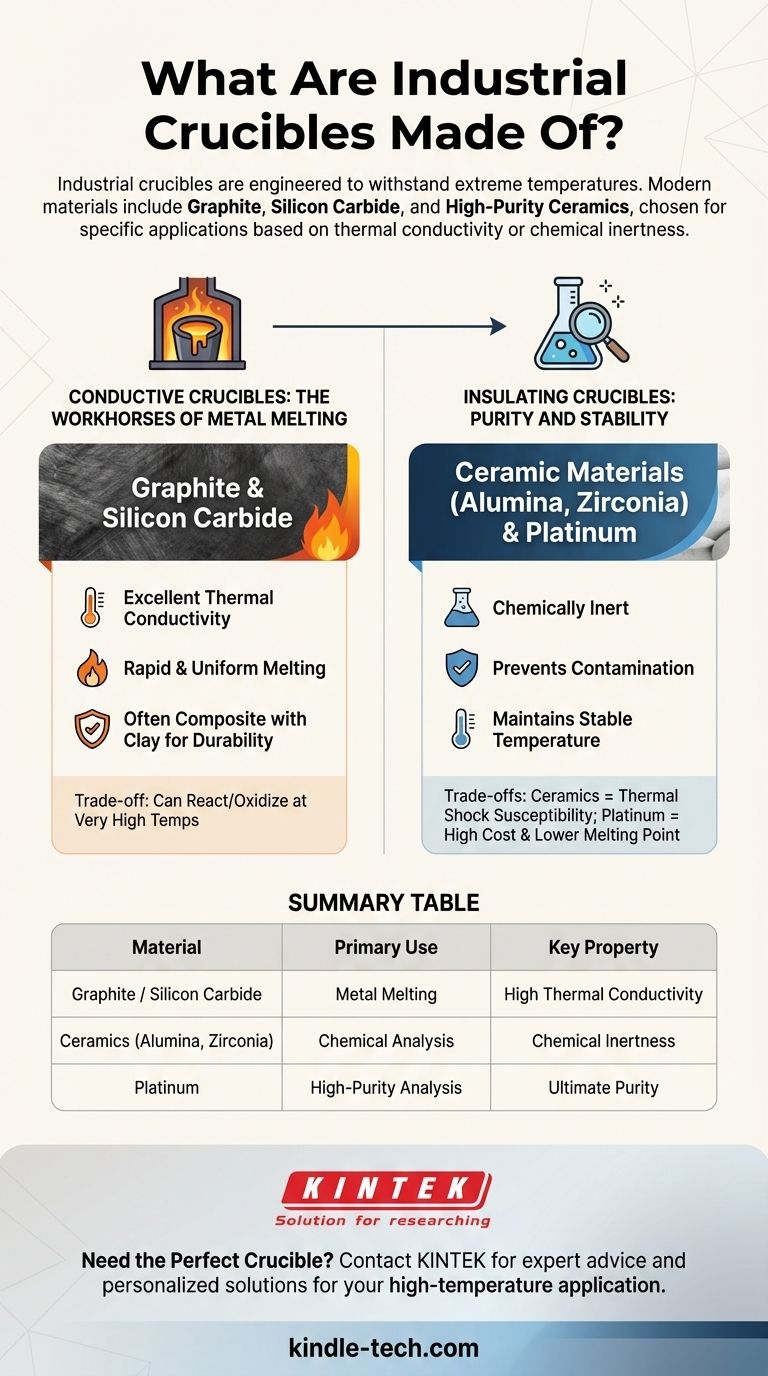
In short, industrial crucibles are made from materials engineered to withstand extreme temperatures, most commonly graphite, silicon carbide, and various high-purity ceramics. While historically made of clay, modern material selection is a precise decision driven entirely by the crucible's intended application, such as whether it needs to conduct heat for melting metals or insulate a sample for chemical analysis.
The most critical factor in choosing a crucible material is not just temperature resistance, but its role in the process. The choice boils down to a fundamental trade-off: conductive materials like graphite for efficient melting versus insulating and inert materials like ceramics for purity and containment.

The Fundamental Divide: Conductive vs. Insulating
The vast array of crucible materials can be understood by separating them into two primary functional categories. This distinction dictates which material is used for which industrial or laboratory task.
Conductive Crucibles: The Workhorses of Metal Melting
Conductive crucibles are designed to efficiently transfer heat from an external source (like a furnace) to the material inside. This makes them essential for foundries and metal casting operations.
The most common materials are graphite and silicon carbide. These materials possess excellent thermal conductivity, allowing for rapid and uniform melting of metals.
They are often manufactured as composites, blending graphite and silicon carbide with other materials like clay to enhance durability and resistance to oxidation.
Insulating Crucibles: Purity and Stability
Insulating crucibles are designed to contain a substance while resisting chemical interaction and maintaining a stable temperature, rather than actively transferring heat.
These are typically made from ceramic materials like alumina, zirconia, or magnesium oxide. Their primary benefit is being chemically inert, which is critical in laboratory settings.
In a lab, using an inert crucible made of a material like platinum or high-purity ceramic ensures that the crucible itself does not contaminate the chemical sample, which would compromise the results of an analysis.
Understanding the Trade-offs
No single material is perfect for every application. The selection of a crucible involves balancing performance, cost, and lifespan based on the specific high-temperature process.
Graphite & Silicon Carbide: Efficiency vs. Reactivity
These materials offer unparalleled heat transfer, making them ideal for melting. However, they can react with certain molten metals or oxidize and degrade if exposed to air at very high temperatures without a protective glaze.
Ceramics: Purity vs. Thermal Shock
Ceramic crucibles provide superior chemical inertness and can withstand higher temperatures than many graphite-based options. Their main weakness is a susceptibility to thermal shock—cracking if heated or cooled too quickly.
High-Purity Metals (Platinum): Ultimate Purity vs. Cost
For exacting laboratory analysis where any contamination is unacceptable, crucibles made of precious metals like platinum are the standard. They are extremely inert but are prohibitively expensive and have lower melting points than ceramics, making them completely unsuitable for industrial metal casting.
Making the Right Choice for Your Goal
Your application's primary goal will always determine the correct material.
- If your primary focus is efficient, large-scale metal melting: Graphite or silicon-carbide crucibles are the industry standard for superior thermal conductivity.
- If your primary focus is high-purity sample analysis: A platinum or high-purity ceramic crucible is essential to prevent chemical contamination.
- If your primary focus is containing a specialized, high-temperature reaction: An insulating ceramic crucible chosen for its chemical compatibility is the correct choice.
Ultimately, selecting the right crucible is about matching the material's physical and chemical properties to the precise demands of your high-temperature application.
Summary Table:
| Material | Primary Use | Key Property |
|---|---|---|
| Graphite / Silicon Carbide | Metal Melting | High Thermal Conductivity |
| Ceramics (Alumina, Zirconia) | Chemical Analysis | Chemical Inertness |
| Platinum | High-Purity Analysis | Ultimate Purity |
Need the Perfect Crucible for Your Application?
KINTEK specializes in high-performance lab equipment and consumables. Whether you need a conductive crucible for efficient metal melting or an inert ceramic crucible for precise chemical analysis, our experts will help you select the ideal material for your specific process, ensuring optimal performance, purity, and cost-effectiveness.
Contact our team today to discuss your crucible requirements and get a personalized solution!
Visual Guide
Related Products
- High Purity Pure Graphite Crucible for Electron Beam Evaporation
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- High Purity Pure Graphite Crucible for Evaporation
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- What are crucibles used for? A Guide to High-Temperature Containers for Melting & Analysis
- Why is the use of high-purity alumina crucibles necessary for NMC powders? Ensure Purity in Cathode Synthesis
- Why are high-purity alumina crucibles selected for oxidation tests? Ensure Precision at 1200°C for Fe-Cr-Al ODS Alloys
- How long do crucibles last? Extend crucible lifespan from 15 to 100+ uses
- What makes high-purity alumina crucibles suitable for pack cementation? Optimize Coating Purity & Thermal Stability
- What is the function of a high-purity alumina crucible in the aluminizing process? Ensure Pure Coating Reactions
- What is the role of a boat-shaped alumina crucible? Optimize LATP Solid-State Electrolyte Synthesis Efficiency
- Why are alumina crucibles or baskets essential for Boudouard reaction studies? Ensure Pure Data & Chemical Inertness



















