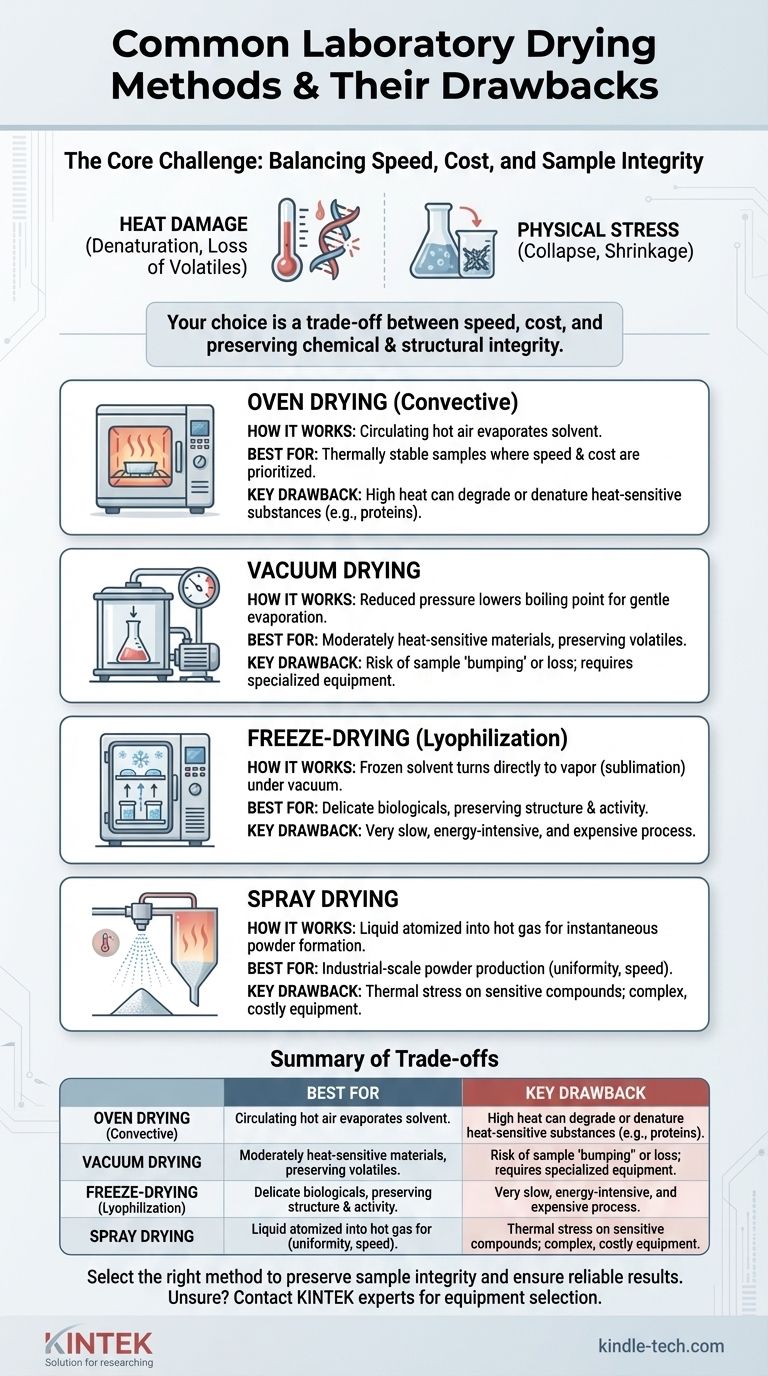
The most common laboratory drying methods are oven drying, vacuum drying, and spray drying, with their primary drawback being the potential for heat and physical stress to alter a sample's fundamental properties. This damage can manifest as denatured proteins, loss of volatile compounds, or changes in the material's final size and texture, compromising the integrity of your results.
The central challenge in laboratory drying is not merely removing a solvent, but doing so without destroying the sample. Your choice of method represents a critical trade-off between speed, cost, and the preservation of your material's chemical and structural integrity.

Why Drying Method Matters: Preserving Sample Integrity
The goal of drying is to remove a liquid solvent, typically water, from a solid sample. The challenge is that the methods used to accelerate this process can inflict irreversible damage.
The two primary sources of damage during drying are heat and physical stress. Heat can break down heat-sensitive compounds, while the physical process of evaporation can cause delicate structures to collapse, fundamentally changing the sample.
A Breakdown of Common Laboratory Techniques
Each drying technique offers a different balance of speed, gentleness, and cost. Understanding how each one works is key to selecting the appropriate method for your specific sample and analytical goal.
Oven Drying (Convective Drying)
This is the simplest method, involving placing a sample in an oven with circulating hot air. The heat provides the energy for the solvent to evaporate, and the air circulation carries the vapor away.
It is best suited for samples that are thermally stable, where speed and simplicity are more important than preserving a delicate structure.
The significant drawback is that direct, high heat can easily degrade or denature heat-sensitive substances like proteins, pharmaceuticals, or certain polymers, completely altering their properties as noted in the references.
Vacuum Drying
This method involves placing the sample in a chamber and reducing the pressure. Lowering the ambient pressure reduces the boiling point of the solvent, allowing for rapid evaporation at a much lower temperature than in a standard oven.
Vacuum drying is a significant improvement for moderately heat-sensitive materials. It is gentler than oven drying and is often used for chemicals or food products where preserving volatile flavor and aroma compounds is important.
However, the rapid boiling under vacuum can cause some samples to "bump" or splash, leading to sample loss. It also requires more specialized and expensive equipment than a simple oven.
Freeze-Drying (Lyophilization)
Freeze-drying is the gold standard for delicate materials. The sample is first frozen solid, then placed under a deep vacuum, causing the frozen solvent to turn directly into vapor without passing through a liquid phase (sublimation).
This is by far the gentlest drying method. Because it avoids liquid evaporation and high heat, it excels at preserving the structure of biological materials like proteins, enzymes, microbes, and tissues. The final product is often light, porous, and easily rehydrated.
The primary disadvantages are that it is a very slow and energy-intensive process, requiring expensive, specialized equipment.
Spray Drying
Spray drying is a continuous process where a liquid sample is atomized into fine droplets inside a hot gas chamber. The high surface area of the droplets allows for nearly instantaneous evaporation of the solvent, leaving behind a dry powder.
This technique is extremely fast and scalable, making it ideal for industrial production of powders like milk, coffee, and pharmaceuticals. It produces consistent, uniform particles.
While the exposure time to heat is very short, the high temperatures can still cause thermal stress on sensitive compounds. The equipment is also complex and represents a significant capital investment.
Understanding the Trade-offs
Choosing a method blindly can lead to failed experiments and wasted materials. The key is to understand the compromises you are making.
The Impact of Heat
As mentioned in the references, heat is the most common culprit for sample damage. It can denature proteins, changing their shape and rendering them inactive. It can also break down chemical compounds or cause the loss of volatile components that contribute to a substance's characteristics.
The Impact of Physical Stress
The transition from liquid to gas creates physical forces. As water evaporates from a porous sample, surface tension can cause delicate internal structures to collapse, leading to a shrunken, hardened final product with entirely different properties. Freeze-drying is the only common method that completely avoids this issue.
The Cost and Complexity Factor
A simple lab oven is inexpensive and easy to operate. In contrast, a vacuum oven is more complex, and a freeze-dryer or spray dryer represents a major investment in both equipment and operator training. Your choice is often constrained by available resources.
Making the Right Choice for Your Sample
Your decision must be driven by the nature of your sample and the goal of your work.
- If your primary focus is speed and cost for thermally stable materials: Oven drying is the most practical and efficient choice.
- If your primary focus is preserving moderately heat-sensitive compounds: Vacuum drying offers a good balance of speed and gentleness.
- If your primary focus is maintaining the biological activity and delicate structure of your sample: Freeze-drying (lyophilization) is the unequivocal best option, despite its cost and time.
- If your primary focus is creating a uniform powder from a liquid at an industrial scale: Spray drying is the industry standard for its speed and consistency.
Ultimately, selecting the correct drying method is a foundational step toward achieving reliable and reproducible scientific results.
Summary Table:
| Method | Best For | Key Drawback |
|---|---|---|
| Oven Drying | Thermally stable samples; speed & cost | High heat can degrade sensitive materials |
| Vacuum Drying | Moderately heat-sensitive compounds | Can cause sample 'bumping' and loss |
| Freeze-Drying | Delicate biologicals; structure preservation | Slow, energy-intensive, and expensive |
| Spray Drying | Industrial-scale powder production | Thermal stress on sensitive compounds |
Unsure which drying method is right for your specific application? The wrong choice can lead to denatured proteins, loss of volatile compounds, and compromised results. KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the ideal equipment—from robust ovens to gentle freeze-dryers—to preserve your sample's integrity and ensure the reliability of your data. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
People Also Ask
- What role does freeze-drying play in biotechnology? Preserving Biological Integrity for Long-Term Stability
- What are the characteristics of crystalline materials in lyophilization? Master Crystal Size & Eutectic Temperature
- What is the eutectic point in lyophilization? Master the Critical Temperature for Success
- What are the key components of a Laboratory Freeze Dryer? Understand the 4 Core Systems for Successful Lyophilization
- What types of samples are ideal for freeze-drying? Preserve Delicate Biologicals and Materials



















