From a practical standpoint, the primary advantage of wet ashing is its speed and ability to retain volatile elements that would be lost in other methods. However, its main disadvantages are the significant risk of sample contamination from the chemical reagents used and the safety hazards associated with handling hot, concentrated acids.
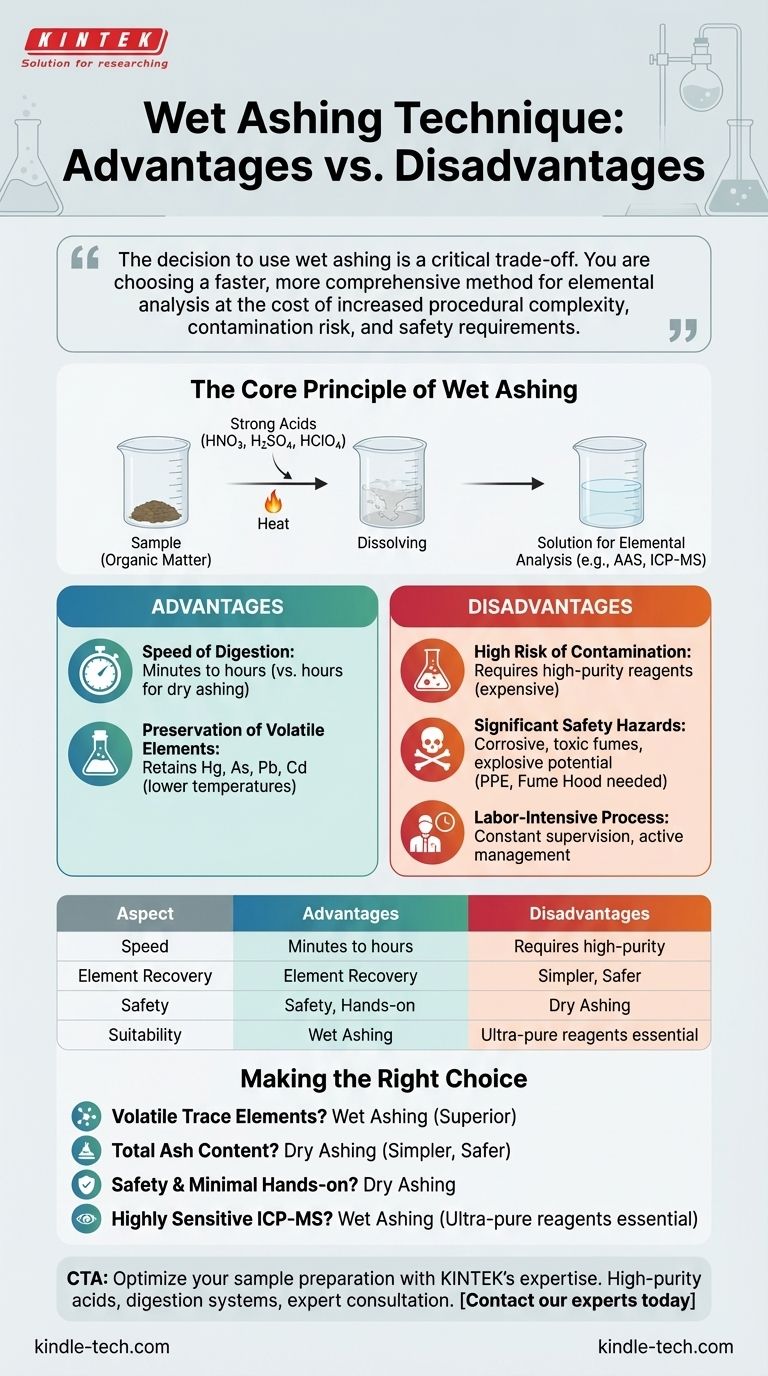
The decision to use wet ashing is a critical trade-off. You are choosing a faster, more comprehensive method for elemental analysis at the cost of increased procedural complexity, contamination risk, and safety requirements.

The Core Principle of Wet Ashing
What is Wet Ashing?
Wet ashing, also known as acid digestion, is a sample preparation technique. It uses a mixture of strong acids (such as nitric, sulfuric, or perchloric acid) and heat to chemically decompose the organic matter of a sample.
The ultimate goal is not to measure total ash, but to dissolve the sample matrix completely. This leaves the inorganic elements of interest suspended in a liquid solution, ready for analysis.
The Goal: Preparing for Elemental Analysis
This resulting acidic solution is the perfect format for modern analytical instruments.
Techniques like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma Mass Spectrometry (ICP-MS) require liquid samples to measure the concentration of specific minerals and trace metals.
Key Advantages of the Wet Ashing Method
Speed of Digestion
Compared to its alternative, dry ashing, the wet ashing process is significantly faster.
A complete digestion can often be achieved in a period ranging from ten minutes to a few hours, whereas dry ashing in a muffle furnace typically requires many hours or is run overnight.
Preservation of Volatile Elements
This is arguably the most critical advantage of wet ashing. High-temperature dry ashing can cause volatile elements like mercury, arsenic, lead, and cadmium to be lost to the atmosphere, leading to inaccurate results.
Wet ashing uses much lower temperatures, typically determined by the boiling point of the acids used. This ensures these sensitive elements are retained in the solution for accurate quantification.
Understanding the Trade-offs and Disadvantages
High Risk of Contamination
The largest drawback of wet ashing is the potential for introducing contaminants. The strong acids and reagents used must be of extremely high purity, as any trace metals within them will be added to the sample.
This can artificially inflate the measured concentration of the elements you are trying to analyze, compromising the integrity of your results. Using ultra-pure, trace-metal grade acids is essential but also expensive.
Significant Safety Hazards
Working with hot, concentrated acids is inherently dangerous. The process generates corrosive and toxic fumes, mandating the use of a specialized fume hood and appropriate personal protective equipment (PPE).
Certain acids, like perchloric acid, can become explosive under specific conditions, requiring highly trained personnel and careful handling protocols.
Labor-Intensive Process
Unlike the "set it and forget it" nature of placing samples in a muffle furnace for dry ashing, wet ashing requires constant supervision.
An analyst must actively manage the process, adding reagents and monitoring the digestion to prevent samples from boiling dry or reacting too violently.
Making the Right Choice for Your Analysis
Your choice of method should be dictated entirely by your analytical objective and the resources available.
- If your primary focus is analyzing for volatile trace elements (like mercury or arsenic): Wet ashing is the superior and often the only viable method to ensure accurate results.
- If your primary focus is determining the total mineral (ash) content of a sample: Traditional dry ashing is simpler, safer, and perfectly suitable for this purpose.
- If your primary focus is safety and minimizing hands-on time: Dry ashing is less labor-intensive and avoids the direct handling of hazardous liquid acids.
- If your primary focus is preparing a sample for highly sensitive ICP-MS analysis: Wet ashing is necessary, but you must invest in ultra-pure reagents to avoid critical contamination.
Understanding these fundamental trade-offs empowers you to select the sample preparation technique that ensures the accuracy and reliability of your final results.
Summary Table:
| Aspect | Advantages | Disadvantages |
|---|---|---|
| Speed | Fast digestion (minutes to hours) | Labor-intensive, requires constant supervision |
| Element Recovery | Retains volatile elements (e.g., Hg, As) | High risk of contamination from reagents |
| Safety | Lower temperatures than dry ashing | Hazardous acid handling, toxic fumes |
| Suitability | Ideal for ICP-MS, AAS analysis | Requires high-purity, expensive acids |
Optimize your sample preparation with KINTEK's expertise.
Choosing the right ashing method is critical for accurate elemental analysis. Whether your priority is the speed and precision of wet ashing or the simplicity of dry ashing, KINTEK provides the high-quality lab equipment and consumables you need for reliable results.
We specialize in serving laboratory needs with:
- High-purity acids and reagents to minimize contamination risk.
- Robust digestion systems and safety equipment (like fume hoods) to protect your team.
- Expert consultation to help you select the ideal method for your specific analysis of volatile trace elements or total ash content.
Ensure the integrity of your analysis. Contact our experts today to discuss your lab's requirements and find the perfect solution!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the methods of ash analysis? Dry Ashing, Wet Ashing, and More Explained
- What controls melting point? The Hierarchy of Forces from Ionic Bonds to Intermolecular Attractions
- What is the melting temperature of ceramics? Understanding High-Temperature Material Performance
- How is the ash content determined in a muffle furnace? Master the Gravimetric Analysis Method
- What is the difference between a muffle furnace and a normal furnace? Ensuring Sample Purity with Indirect Heating



















