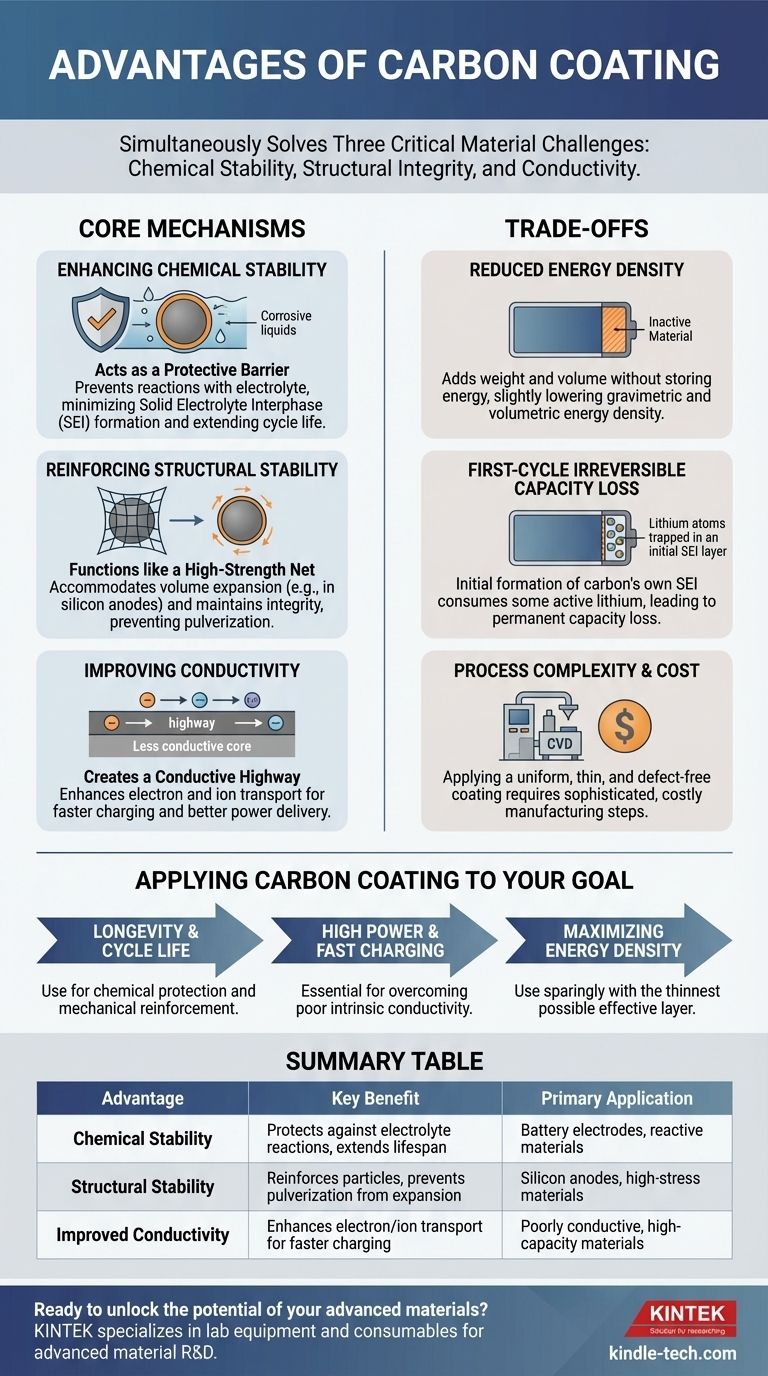
Fundamentally, the advantages of carbon coating are its ability to simultaneously solve three critical material challenges. It enhances chemical stability by acting as a protective barrier, reinforces structural integrity to prevent physical breakdown, and improves the electrical and ionic conductivity of the underlying material.
Carbon coating is rarely just a simple protective layer. It is a multi-functional interface engineered to dramatically improve the performance and lifespan of a core material, especially in demanding applications like battery electrodes.

The Core Mechanisms of Carbon Coating
To understand its advantages, you must first understand the problems it solves at a microscopic level. The benefits of a carbon coating are not isolated; they are interconnected results of a few key physical and chemical mechanisms.
Enhancing Chemical Stability
Many high-performance materials, particularly in energy storage, are chemically reactive with their environment. A carbon coating provides a crucial protective shield.
In lithium-ion batteries, for example, electrode materials can react with the liquid electrolyte. This unwanted reaction forms a resistive layer called the Solid Electrolyte Interphase (SEI), which consumes active lithium and hinders performance over time.
A well-designed carbon coating acts as a physical and chemical barrier. It prevents direct contact between the active material and the electrolyte, minimizing these parasitic reactions and significantly extending the battery's cycle life and stability.
Reinforcing Structural Stability
Mechanical failure is a common limitation for many advanced materials. During operation, some materials undergo significant physical changes.
Consider silicon, a promising anode material for batteries. It experiences massive volume expansion (up to 300%) as it absorbs lithium ions during charging, and contracts upon discharge. This repeated stress can cause the material to pulverize and lose electrical contact.
A conformal carbon coating functions like a flexible, high-strength net. It physically holds the particles together, accommodates the volume changes, and maintains the electrode's structural integrity, preventing catastrophic failure over hundreds of cycles.
Improving Electrical and Ionic Conductivity
Many materials with excellent storage capacity or other desirable properties are unfortunately poor conductors of electrons and ions. This inherent high resistance limits their real-world performance, leading to slow charging and poor power delivery.
Carbon, particularly in its amorphous or graphitic forms, is an excellent electrical conductor. Applying a thin carbon layer creates a conductive highway for electrons to travel across the surface of the non-conductive particles.
Furthermore, this coating can create a more favorable interface for ions (like Li+) to move in and out of the active material. By improving both electronic and ionic conductivity, carbon coating directly translates to higher rate capability (faster charging/discharging) and better overall efficiency.
Understanding the Trade-offs
While highly effective, carbon coating is not a perfect solution and involves critical engineering trade-offs. Objectivity requires acknowledging its potential downsides.
Reduced Energy Density
Carbon itself is typically an "inactive" material in this context; it doesn't store energy in the same way the core material does. Therefore, adding a carbon coating increases the overall weight and volume of the electrode without contributing to its primary function.
This means there is a slight penalty in gravimetric and volumetric energy density. The goal is always to use the thinnest possible coating that still provides the necessary mechanical and chemical benefits.
First-Cycle Irreversible Capacity Loss
During the very first charging cycle of a battery, the carbon coating itself can react with the electrolyte to form its own SEI layer. This process consumes a small amount of active lithium, which is then permanently lost.
This phenomenon, known as first-cycle irreversible capacity loss, slightly reduces the battery's usable capacity from the outset. This loss must be accounted for in the overall cell design.
Process Complexity and Cost
Applying a uniform, thin, and high-quality carbon coating is a sophisticated manufacturing step. Techniques like chemical vapor deposition (CVD) or hydrothermal carbonization add complexity, time, and cost to the production process.
Achieving a perfect coating without defects or inconsistencies at a large scale remains a significant engineering challenge.
Applying Carbon Coating to Your Goal
Your decision to use carbon coating should be driven by the primary problem you are trying to solve.
- If your primary focus is longevity and cycle life: Carbon coating is your most effective tool for preventing chemical degradation and accommodating mechanical stress like volume expansion.
- If your primary focus is high power and fast charging: Carbon coating is essential for overcoming the poor intrinsic conductivity of many high-capacity materials.
- If your primary focus is maximizing energy density: You must use carbon coating sparingly, optimizing for the thinnest possible layer that achieves the minimum required stability and conductivity.
Ultimately, carbon coating is a foundational technique for unlocking the true potential of otherwise limited advanced materials.
Summary Table:
| Advantage | Key Benefit | Primary Application |
|---|---|---|
| Chemical Stability | Protects against electrolyte reactions, extends lifespan | Battery electrodes, reactive materials |
| Structural Stability | Reinforces particles, prevents pulverization from expansion | Silicon anodes, high-stress materials |
| Improved Conductivity | Enhances electron/ion transport for faster charging | Poorly conductive, high-capacity materials |
Ready to unlock the potential of your advanced materials?
Carbon coating is a foundational technique for enhancing performance and longevity, but its success depends on precise application. KINTEK specializes in lab equipment and consumables for advanced material R&D, including solutions for developing and testing carbon coatings.
Whether you're optimizing battery electrodes or improving material stability, our expertise can help you achieve superior results. Contact our experts today to discuss how we can support your specific laboratory needs and drive your projects forward.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- Why are specialized battery research tools necessary for evaluating recycled graphite? Ensure Material Validation
- What is carbon coating? Enhance Battery Performance with a Conductive Shield
- What role does powder mixing equipment play in composite cathodes? Optimize Nb2O5/LPSC/CNF Battery Performance
- Why use a double-stage rotary vane vacuum pump for lithium foil drying? Ensure Chemical Purity and Thermal Stability
- What is the primary function of a vacuum drying oven in LiFePO4 cathode preparation? Ensure High Battery Performance
- How do precision coating and multi-stage thermal treatment ensure supercapacitor electrode performance? Expert Guide
- What are carbon cloths used for? Conquer Static Dust on Electronics & Screens
- What are the real life applications of graphite? Powering Batteries, Industry, and Technology