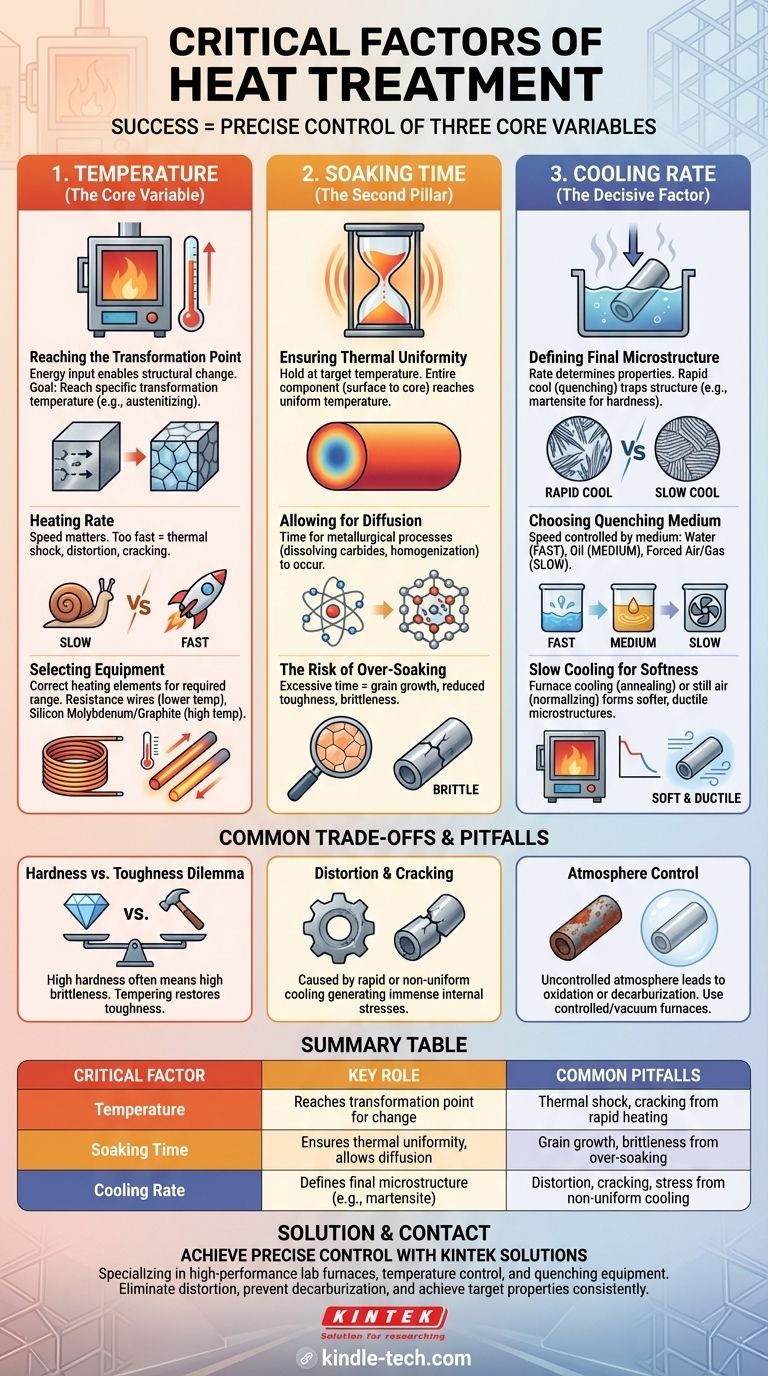
In heat treatment, success is a function of precise control over three core variables. The most critical factors are the heating temperature reached, the soaking time held at that temperature, and the subsequent cooling rate. These three elements are not independent; they work in concert to fundamentally transform a material's internal microstructure, which in turn dictates its final mechanical properties like hardness, strength, and ductility.
Heat treatment is not simply about heating a material; it is a controlled metallurgical process. The precise interplay between temperature, time, and cooling rate is what allows you to engineer a material's final properties to meet specific performance demands.

The Core Variable: Temperature
The temperature to which a material is heated is the starting point and perhaps the most fundamental factor. It is the energy input that enables structural change.
Reaching the Transformation Point
For most steels and many alloys, the goal of heating is to reach a specific "transformation temperature" (such as the austenitizing temperature). At this point, the material's crystal structure changes into a state that is receptive to modification upon cooling.
The Role of Heating Rate
The speed at which this temperature is reached is also important. Heating a component too quickly can induce thermal shock and internal stresses, leading to distortion or even cracking, especially in complex geometries or large cross-sections.
Selecting the Right Equipment
Achieving and maintaining a stable target temperature requires the correct furnace equipment. The heating elements within the furnace must be chosen based on the required temperature range. For instance, common resistance wires are suitable for lower temperatures, while silicon molybdenum rods or graphite elements are necessary for very high-temperature processes.
The Second Pillar: Soaking Time
Once the material reaches the target temperature, it must be held there for a specific duration. This period is known as the soak time.
Ensuring Thermal Uniformity
The first goal of soaking is to ensure the entire component, from its surface to its core, reaches a uniform temperature. Thicker sections naturally require longer soaking times than thinner ones.
Allowing for Diffusion
Metals are not static, especially at high temperatures. Soaking provides the necessary time for metallurgical processes like the dissolution of carbides and the homogenization of alloying elements to occur through diffusion. This ensures the material is in a uniform chemical and structural state before cooling.
The Risk of Over-Soaking
While necessary, excessive soaking time can be detrimental. It can lead to undesirable grain growth within the material's microstructure, which can reduce toughness and make the material brittle.
The Decisive Factor: Cooling Rate
The rate at which the material is cooled from the soaking temperature is often the most decisive factor in determining its final properties.
Defining the Final Microstructure
For hardening steels, a rapid cool (known as quenching) is used to "trap" the high-temperature crystal structure, forcing it to transform into a very hard and strong, but brittle, microstructure called martensite.
Choosing the Quenching Medium
The speed of the quench is controlled by the medium used. Water provides a very fast quench, oil is slower and less severe, and forced air or inert gas is slower still. The correct medium depends on the material's "hardenability"—its ability to form martensite.
Slow Cooling for Softness
Conversely, slow cooling—such as letting the part cool down in the furnace (annealing) or in still air (normalizing)—allows the crystal structure to transform into softer, more ductile microstructures like ferrite and pearlite.
Understanding the Trade-offs and Pitfalls
Controlling these factors is a balancing act, and misunderstanding their interplay can lead to failed parts.
The Hardness vs. Toughness Dilemma
The most common trade-off in heat treatment is between hardness and toughness. A very fast quench may produce extreme hardness, but it often comes at the cost of brittleness and an increased risk of cracking. Subsequent tempering is often required to restore some toughness.
The Danger of Distortion and Cracking
Rapid or non-uniform cooling is the primary cause of parts warping, distorting, or cracking. This is due to the immense internal stresses generated as different sections of the part cool and transform at different rates.
The Importance of Atmosphere Control
The atmosphere inside the furnace is a silent but critical factor. An uncontrolled atmosphere can lead to oxidation (scaling) or the loss of carbon from the surface (decarburization), both of which can ruin a component. Controlled atmospheres or vacuum furnaces are used to prevent these reactions.
Applying These Factors to Your Goal
The ideal combination of temperature, time, and cooling is entirely dependent on your desired outcome.
- If your primary focus is maximum hardness (e.g., for cutting tools): You will need to achieve the correct austenitizing temperature followed by a quench rapid enough to form a fully martensitic structure.
- If your primary focus is improving machinability (e.g., annealing): The key is heating to the correct temperature and then ensuring a very slow, controlled cooling rate to produce the softest possible microstructure.
- If your primary focus is relieving internal stress (e.g., from welding or machining): The goal is a lower temperature hold followed by slow cooling, where the cooling rate is managed to avoid reintroducing new stresses.
Mastering these core factors transforms heat treatment from a simple heating process into a precise engineering tool.
Summary Table:
| Critical Factor | Key Role | Common Pitfalls |
|---|---|---|
| Temperature | Reaches transformation point for microstructural change | Thermal shock, cracking from rapid heating |
| Soaking Time | Ensures thermal uniformity and allows for diffusion | Grain growth and brittleness from over-soaking |
| Cooling Rate | Defines final microstructure (e.g., martensite for hardness) | Distortion, cracking, and stress from non-uniform cooling |
Achieve precise control over your heat treatment processes with KINTEK's expert solutions.
Whether you are developing cutting tools requiring maximum hardness or annealing parts for improved machinability, the precise interplay of temperature, time, and cooling is critical. KINTEK specializes in high-performance lab furnaces, temperature control systems, and quenching equipment designed to deliver the stability and uniformity your processes demand.
We provide the reliable equipment and technical support to help you:
- Eliminate distortion and cracking with uniform heating and controlled cooling.
- Prevent decarburization and scaling using advanced atmosphere control options.
- Consistently achieve your target material properties, batch after batch.
Ready to transform your heat treatment from a simple process into a precise engineering tool? Contact our experts today to discuss your specific application and discover the right KINTEK solution for your laboratory's needs.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What are vacuum furnace parts? A Guide to the Core Systems for Precision Heat Treatment
- What are the uses of vacuum furnace? Achieve Unmatched Material Purity and Performance
- Can I vacuum the inside of my furnace? A Guide to Safe DIY Cleaning vs. Professional Service
- Why is environmental control within a vacuum furnace important for diffusion bonding? Master Titanium Alloy Laminates
- How does a vacuum heat treatment work? Achieve Superior Material Properties in a Pristine Environment



















