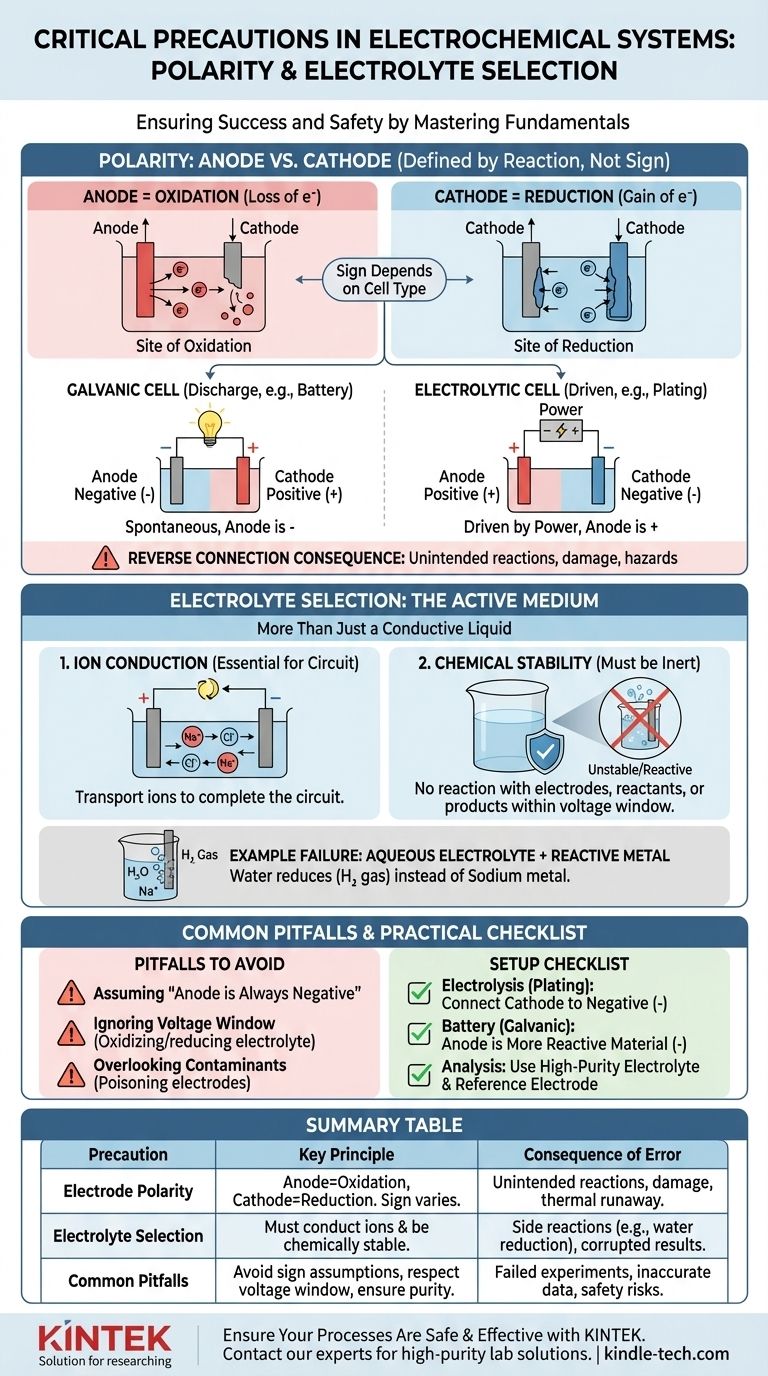
In any electrochemical system, the two most critical precautions are ensuring correct electrode polarity and selecting the proper electrolyte for your reaction. Polarity dictates which chemical process occurs where, while the electrolyte provides the necessary medium for that process. Getting either wrong can lead to failed experiments, damaged equipment, or significant safety hazards.
The polarity of your electrodes defines the direction of the chemical reaction, and the electrolyte determines its feasibility. Mismatching either one will fundamentally alter, inhibit, or actively corrupt the intended electrochemical process.
The Critical Role of Polarity: Anode vs. Cathode
Correctly identifying your anode and cathode is the first step in building a functional and predictable electrochemical cell. This goes beyond simply labeling terminals as "positive" or "negative."
What "Polarity" Truly Means
The terms anode and cathode are defined by the reaction type, not by their charge.
- The Anode is always the site of Oxidation (loss of electrons).
- The Cathode is always the site of Reduction (gain of electrons).
The confusion arises because the sign (+ or -) of these electrodes depends on the type of cell. In a battery discharging (a galvanic cell), the anode is the negative terminal. In an electrolysis setup (an electrolytic cell), the anode is connected to the positive terminal of the power supply.
The Consequence of Reverse Connection
Connecting the electrodes incorrectly forces the chemical reactions to run in reverse or in an unintended way.
In an electrolytic cell (e.g., electroplating), a reverse connection means you will strip material from the electrode you intended to plate and deposit material onto your source anode, destroying both.
In a rechargeable battery, charging with reversed polarity can cause irreversible damage to the electrode structures, leading to permanent capacity loss, internal short circuits, and potentially dangerous thermal runaway.
Electrolyte Selection: More Than Just a Conductive Liquid
The electrolyte is not a passive component; it is an active and critical part of the electrochemical system. Its properties dictate what reactions are even possible.
The Primary Function: Ion Conduction
The electrolyte's most basic job is to transport ions between the anode and cathode, completing the electrical circuit. Without a medium for ion flow, the entire reaction would halt instantly.
The Critical Function: Chemical Stability
The electrolyte and its solvent must be chemically inert under the operating conditions. They should not react with the electrodes, the reactants, or the products of your primary reaction. This is the source of the "unwanted side reactions" mentioned in safety protocols.
Example: Why Aqueous Electrolytes Fail for Reactive Metals
Imagine trying to produce sodium metal by electrolyzing a solution of sodium chloride (NaCl) in water.
You might expect sodium ions (Na+) to move to the cathode, gain an electron, and form sodium metal. However, water (H₂O) is also present and is much easier to reduce than sodium ions. As a result, you will produce hydrogen gas at the cathode, and no sodium metal will form. The electrolyte choice made the desired reaction impossible.
Understanding the Trade-offs and Common Pitfalls
Avoiding simple mistakes requires understanding the underlying principles that govern all electrochemical cells.
Pitfall 1: Assuming "Anode is Negative"
The most common error is misidentifying the anode and cathode. Always remember to define them by the reaction (oxidation/reduction), not by a fixed sign. Determine if your cell is galvanic (spontaneous) or electrolytic (driven by external power) to assign the correct sign.
Pitfall 2: Ignoring the Electrolyte's Voltage Window
Every electrolyte has a potential window of stability. If you apply a voltage that exceeds this window, you will begin to oxidize or reduce the electrolyte itself, not your intended target. This consumes energy, creates impurities, and stops the desired reaction.
Pitfall 3: Overlooking Contaminants
Even trace impurities in an electrolyte can have a massive impact. Contaminants can deposit on an electrode surface, "poisoning" it and preventing the intended reaction, or they can act as catalysts for unwanted side reactions that corrupt your results.
A Practical Checklist for Your Setup
Use these guidelines to ensure your experimental setup is correct from the start.
- If your primary focus is electrolysis (e.g., plating, refining): Connect the electrode you want to plate (the cathode) to the negative terminal of the power supply and the source material (the anode) to the positive terminal.
- If your primary focus is building a battery (a galvanic cell): The more chemically reactive material that gets oxidized is your anode (negative terminal), and the less reactive material is your cathode (positive terminal).
- If your primary focus is accurate electrochemical analysis: Use a high-purity, often degassed electrolyte to prevent side reactions, and confirm your polarity setup relative to a known standard or reference electrode.
By treating polarity and electrolyte choice as fundamental design parameters, you move from simply following instructions to truly engineering your desired electrochemical outcome.

Summary Table:
| Precaution | Key Principle | Consequence of Error |
|---|---|---|
| Electrode Polarity | Anode = Oxidation site; Cathode = Reduction site. Sign depends on cell type (galvanic vs. electrolytic). | Reverse connection causes unintended reactions (e.g., stripping instead of plating), equipment damage, or thermal runaway. |
| Electrolyte Selection | Must conduct ions and remain chemically stable under operating conditions (voltage window). | Wrong electrolyte enables side reactions (e.g., water reduction instead of sodium deposition), corrupts results, and wastes energy. |
| Common Pitfalls | Assuming 'anode is always negative'; ignoring electrolyte voltage window; overlooking contaminants. | Failed experiments, inaccurate data, and safety risks due to unintended chemical processes. |
Ensure Your Electrochemical Processes Are Safe and Effective with KINTEK
Are you setting up an electrolysis, plating, or battery development project? Proper electrode polarity and electrolyte selection are non-negotiable for achieving accurate results and maintaining safety. At KINTEK, we specialize in providing high-purity lab equipment and consumables—including electrochemical cells, pure electrolytes, and reliable power supplies—to support your laboratory's unique needs.
Let us help you avoid costly mistakes and hazards. Contact our experts today to discuss your specific application and discover how KINTEK's solutions can enhance your electrochemical workflow.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- Platinum Auxiliary Electrode for Laboratory Use
- Nickel Aluminum Tabs for Soft Pack Lithium Batteries
People Also Ask
- How should a platinum wire/rod electrode be cleaned before use? A Guide to Reliable Electrochemical Data
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What is the application of RRDE? Unlock Quantitative Catalyst and Reaction Insights
- What is the difference between ring disk electrode and rotating disk electrode? Unlock Deeper Electrochemical Insights
- What is a common application for the platinum wire/rod electrode? The Essential Guide to Counter Electrodes



















