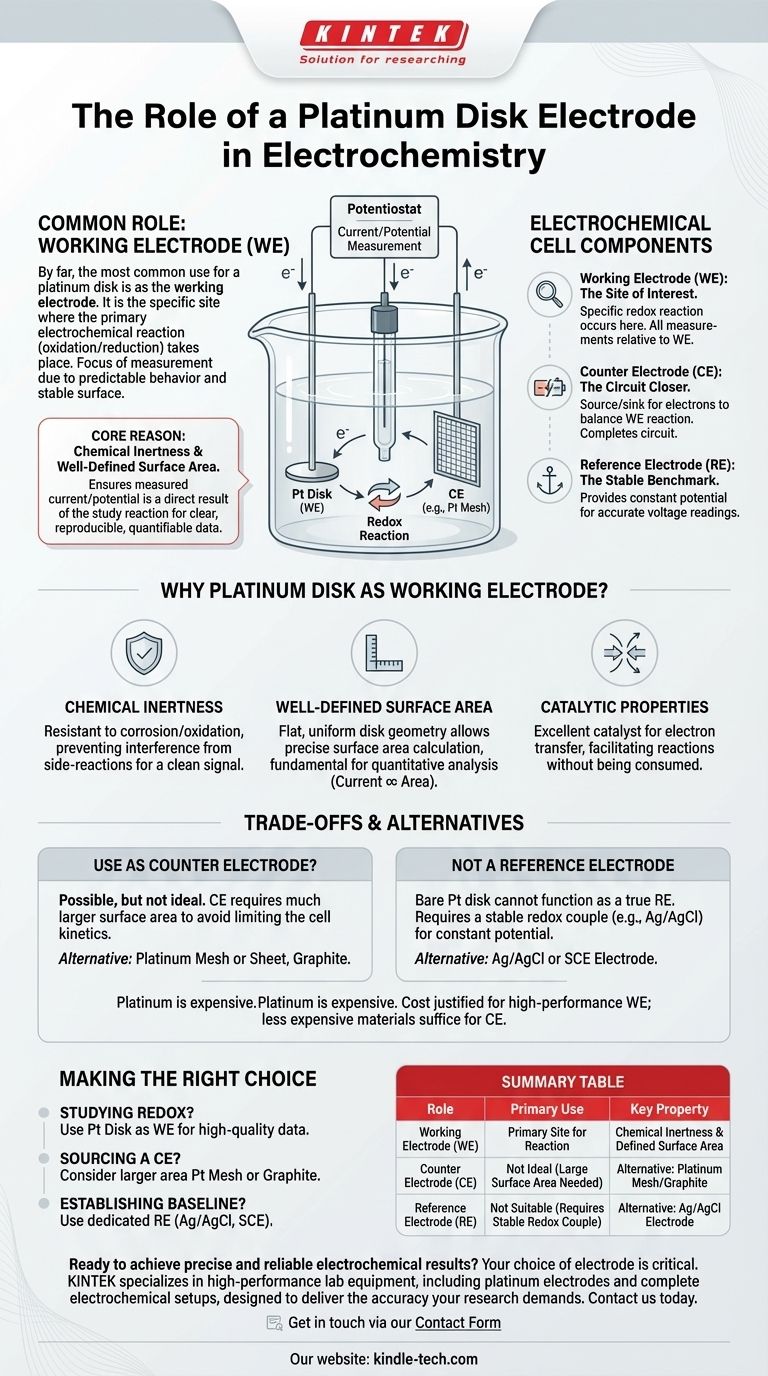
By far, the most common role for a platinum disk electrode in an electrochemical setup is as the working electrode (WE). This is the specific site where the primary electrochemical reaction you intend to study—be it oxidation or reduction—takes place. Its predictable behavior and stable surface make it the focus of the measurement.
The core reason a platinum disk is chosen as the working electrode is its combination of chemical inertness and well-defined surface area. This ensures that the measured current or potential is a direct result of the reaction you are studying, providing clear, reproducible, and quantifiable data.
Understanding the Roles in an Electrochemical Cell
To grasp why the platinum disk is so suited for its role, it's essential to understand the function of each component in a standard three-electrode system.
The Working Electrode (WE): The Site of Interest
The working electrode is the centerpiece of your experiment. It is the surface on which the specific redox reaction you want to analyze occurs. All primary measurements of current and potential are made relative to the state of the WE.
The Counter Electrode (CE): The Circuit Closer
The counter electrode (also called the auxiliary electrode) acts as a source or sink for electrons to balance the reaction happening at the working electrode. Its sole purpose is to pass current and complete the electrical circuit, ensuring the WE's reaction is not limited by a lack of available charge.
The Reference Electrode (RE): The Stable Benchmark
The reference electrode provides a stable, constant potential that does not change regardless of the reactions occurring in the cell. The potential of the working electrode is measured against this stable reference, allowing for accurate and meaningful voltage readings.
The Platinum Disk as the Ideal Working Electrode
A platinum disk is not just a convenient choice for the working electrode; its physical and chemical properties make it uniquely qualified for the job.
Chemical Inertness
Platinum is a noble metal, meaning it is highly resistant to corrosion and oxidation in most electrolyte solutions. This inertness is critical because it ensures the electrode itself does not interfere with or participate in unwanted side-reactions, giving you a clean signal from your intended process.
Well-Defined Surface Area
The "disk" geometry provides a flat, uniform, and easily calculable surface area. Because the measured current is directly proportional to this area, having a known and reproducible surface is fundamental for quantitative electrochemical analysis.
Catalytic Properties
Platinum is an excellent catalyst for a wide range of electron transfer reactions. It facilitates the movement of electrons between the electrode and the species in the solution without being consumed, enabling the study of many different chemical systems.
Understanding the Trade-offs and Alternatives
While the platinum disk excels as a working electrode, it's important to understand its limitations and alternative uses.
Use as a Counter Electrode
A platinum disk can technically be used as a counter electrode. However, it's often not the ideal choice for this role.
Counter electrodes typically require a much larger surface area than the working electrode. Using a larger platinum mesh or sheet prevents the counter electrode's own reaction kinetics from becoming the rate-limiting step of the entire cell.
Not a Reference Electrode
A bare platinum disk cannot function as a true reference electrode. A reference electrode requires a specific, stable redox couple (like Ag/AgCl) to maintain a constant potential. While a platinum wire can sometimes be used as a "pseudo-reference," it is not stable and is unsuitable for any experiment requiring accurate potential measurement.
The Cost Factor
Platinum is an expensive precious metal. This cost is often justified for its use as a high-performance working electrode where precision is paramount. For the less-demanding role of a counter electrode, a less expensive material like graphite or a different form of platinum (like a wire or gauze) is often sufficient.
Making the Right Choice for Your Experiment
Your experimental goal dictates the proper use of your platinum disk electrode.
- If your primary focus is studying a specific redox reaction: Use the platinum disk as your working electrode to ensure high-quality, reproducible data.
- If your primary focus is sourcing a counter electrode: Consider a platinum sheet, mesh, or graphite rod, as their larger surface area is generally more suitable.
- If your primary focus is establishing a potential baseline: You must use a dedicated reference electrode, such as a Silver/Silver Chloride (Ag/AgCl) or Saturated Calomel Electrode (SCE).
Ultimately, understanding the distinct function of each electrode is the key to designing a robust and accurate electrochemical experiment.

Summary Table:
| Role | Primary Use | Key Property |
|---|---|---|
| Working Electrode (WE) | Primary Site for Reaction | Chemical Inertness & Defined Surface Area |
| Counter Electrode (CE) | Not Ideal (Large Surface Area Needed) | Alternative: Platinum Mesh/Graphite |
| Reference Electrode (RE) | Not Suitable (Requires Stable Redox Couple) | Alternative: Ag/AgCl Electrode |
Ready to achieve precise and reliable electrochemical results?
Your choice of electrode is critical to your experiment's success. KINTEK specializes in high-performance lab equipment, including platinum electrodes and complete electrochemical setups, designed to deliver the accuracy and reproducibility your research demands.
Contact us today to discuss your specific needs and let our experts help you select the perfect equipment for your lab. Get in touch via our Contact Form and elevate your electrochemical analysis with KINTEK.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Metal Disc Electrode Electrochemical Electrode
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
People Also Ask
- What is a common application for the platinum wire/rod electrode? The Essential Guide to Counter Electrodes
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- How should a platinum wire/rod electrode be cleaned before use? A Guide to Reliable Electrochemical Data
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis



















