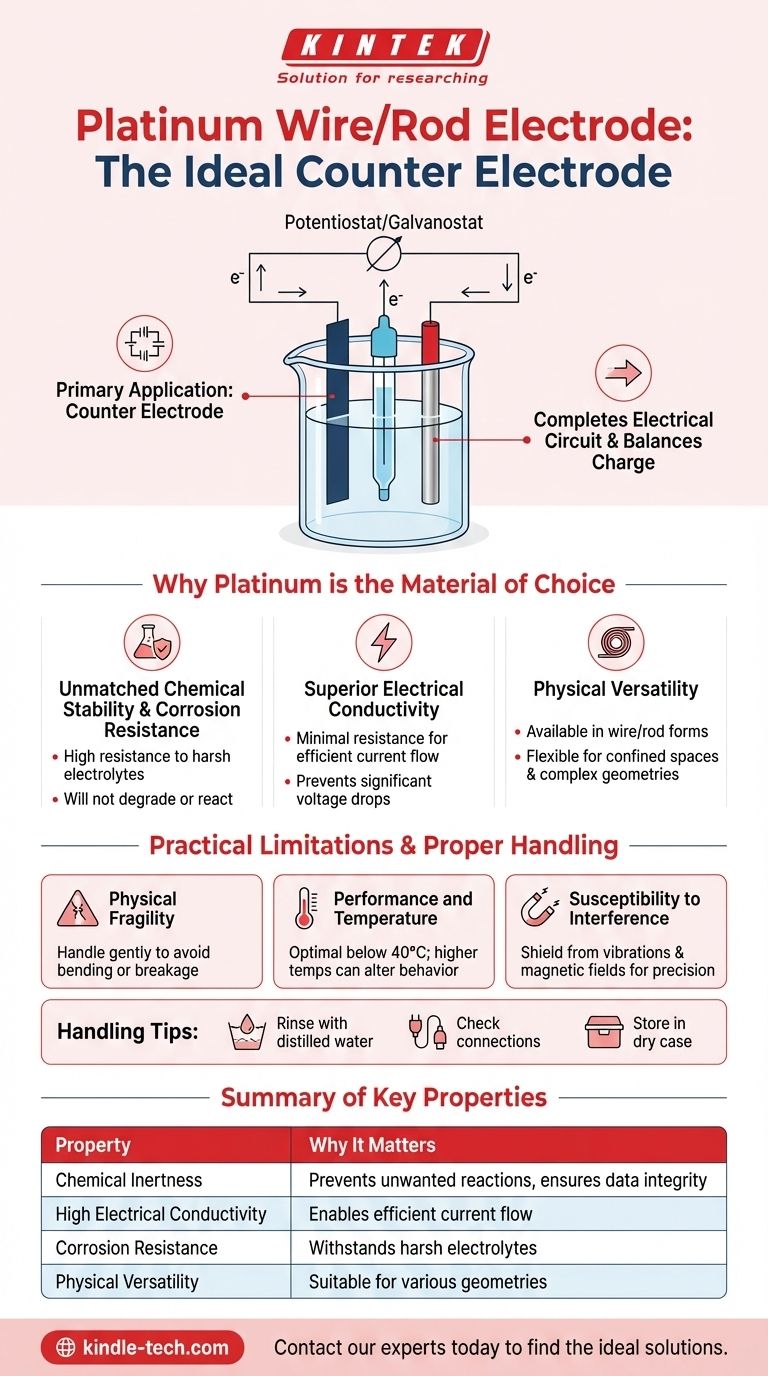
The most common application for a platinum wire or rod electrode is to serve as a counter electrode (also known as an auxiliary electrode) in a standard three-electrode electrochemical cell. Its purpose is to complete the electrical circuit, allowing current to flow to or from the working electrode where the reaction of interest is taking place.
The choice of platinum is deliberate and critical. It is selected for its unique combination of high electrical conductivity and exceptional chemical inertness, which ensures it facilitates the experiment's electrical circuit without interfering with the chemical reaction being studied.

The Role of the Counter Electrode
In electrochemistry, we are typically interested in the reaction occurring at a specific electrode, known as the working electrode. To study this, we need a complete circuit, which requires a second electrode to pass the current through the electrolyte solution.
Completing the Electrical Circuit
The counter electrode's primary function is to serve as a source or sink for electrons. It balances the charge by undergoing a reaction opposite to that of the working electrode, allowing a controlled current or potential to be maintained.
The Critical Need for Inertness
For an experiment to be valid, the counter electrode must be a passive participant. It should not react with the electrolyte or produce byproducts that could diffuse to the working electrode and interfere with the primary measurement. This is where platinum excels.
Why Platinum is the Material of Choice
Platinum's physical and chemical properties make it an ideal, if not the default, choice for a counter electrode in a vast range of experimental setups.
Unmatched Chemical Stability
Platinum is a precious metal renowned for its stability. It exhibits extremely high corrosion resistance and will not degrade or react even in harsh electrolytes like strong acids and alkalis.
Superior Electrical Conductivity
To function effectively, the counter electrode must be able to pass current with minimal resistance. Platinum's excellent electrical conductivity ensures the circuit operates efficiently without introducing significant voltage drops.
Physical Versatility
Platinum wire and rod electrodes can be manufactured in various sizes. The wire form is particularly useful for its flexibility, allowing it to be used in confined spaces or cells with complex geometries.
Understanding the Practical Limitations
While platinum is a superior material, a skilled practitioner must be aware of its practical limitations and handling requirements to ensure accurate results.
Physical Fragility
A thin platinum wire electrode is delicate and can be easily bent or broken. It must be handled gently during setup, cleaning, and storage to avoid damage.
Performance and Temperature
Although the metal itself can withstand very high temperatures, its optimal performance in an electrochemical cell is typically at lower temperatures, often below 40°C. Higher temperatures can alter the behavior of the electrolyte or introduce unwanted side reactions, affecting the electrode's performance and lifespan.
Susceptibility to Interference
For high-precision measurements, the experimental environment must be stable. The electrode system should be shielded from external disturbances like mechanical vibrations or strong magnetic fields that can disrupt the measurement.
Proper Handling for Reliable Results
Correct maintenance is essential for extending the electrode's life and ensuring the integrity of your experimental data.
- If your primary focus is experimental accuracy: Ensure the electrode is clean by rinsing it with distilled water after use and shield your setup from any environmental vibrations or magnetic fields.
- If your primary focus is electrode longevity: Always handle the electrode with care, check wire connections for secure contact, and store it in a dry, protected case to prevent physical damage or corrosion.
By understanding both its ideal properties and its practical limitations, you can leverage the platinum electrode to achieve precise and repeatable electrochemical measurements.
Summary Table:
| Property | Why It Matters for a Counter Electrode |
|---|---|
| Chemical Inertness | Prevents unwanted reactions, ensuring data integrity. |
| High Electrical Conductivity | Enables efficient current flow with minimal resistance. |
| Corrosion Resistance | Withstands harsh electrolytes like strong acids and bases. |
| Physical Versatility | Available in wire/rod forms for various cell geometries. |
Achieve precise and reliable electrochemical results with the right lab equipment.
KINTEK specializes in high-quality lab equipment and consumables, including durable and chemically inert electrodes perfect for your research needs. Whether you are setting up a new electrochemical cell or require reliable components for repeatable experiments, our products are designed to support accuracy and longevity.
Let us help you enhance your lab's capabilities. Contact our experts today to find the ideal solutions for your specific application.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
People Also Ask
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What is the application of RRDE? Unlock Quantitative Catalyst and Reaction Insights
- How should a platinum wire/rod electrode be cleaned before use? A Guide to Reliable Electrochemical Data
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis



















