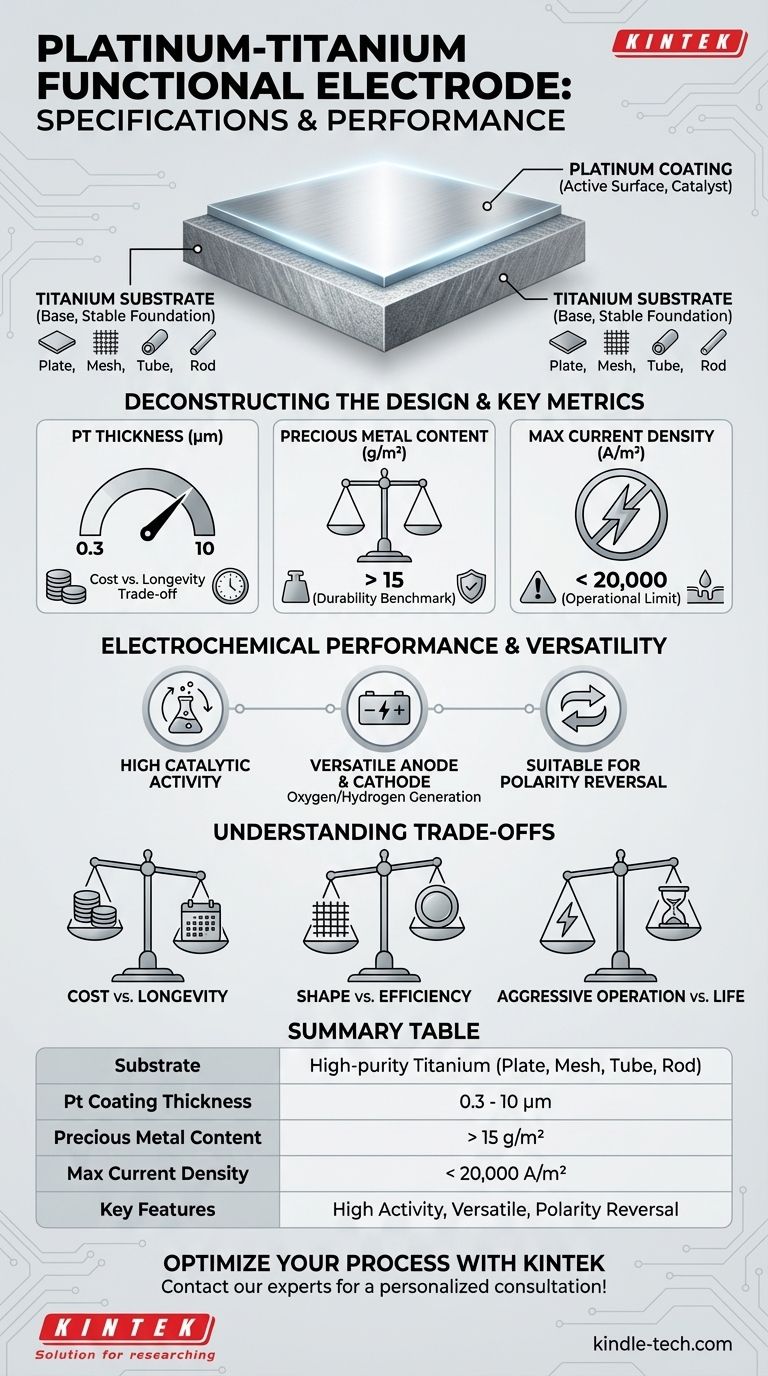
The specifications for a Platinum-Titanium Functional Electrode are defined by its core substrate, the platinum coating, and its operational limits. The substrate is a high-purity titanium form (plate, mesh, tube, or rod) coated with a platinum (Pt) layer between 0.3 and 10 micrometers (μm) thick. Key performance metrics include a precious metal content exceeding 15 grams per square meter (g/m²) and an applicable current density of less than 20,000 amperes per square meter (A/m²).
At its core, this electrode leverages the exceptional corrosion resistance of a titanium substrate with the superior catalytic activity of a platinum surface. The result is a highly durable and versatile component, whose longevity and effectiveness are directly determined by the thickness of the platinum coating and the conditions under which it operates.

Deconstructing the Electrode's Design
To understand this electrode's capabilities, you must understand its individual components and what each specification implies for performance.
The Titanium Substrate: A Stable Foundation
The choice of titanium as the substrate is not arbitrary; it provides the electrode's structural integrity and passive corrosion resistance. Using high-purity titanium ensures there are no impurities that could interfere with the electrochemical process or cause premature failure.
The ability to form the substrate into a plate, mesh, tube, or rod allows the electrode to be customized for specific reactor designs and flow dynamics.
The Platinum Coating: The Active Surface
The platinum (Pt) layer is the functional heart of the electrode. Platinum is an excellent catalyst, meaning it efficiently speeds up electrochemical reactions without being consumed.
The plating thickness, ranging from 0.3 to 10μm, is the most critical variable for balancing cost and lifespan. A thinner layer is less expensive but will wear away faster, while a thicker layer provides a longer service life in more demanding conditions.
Precious Metal Content: A Measure of Durability
The specification of > 15g/m² of precious metal content serves as a quality benchmark. It ensures a sufficient amount of platinum is present on the surface to provide robust and lasting catalytic activity. A higher value generally correlates with a more durable and longer-lasting electrode.
Current Density: The Operational Limit
The applicable current of < 20,000A/m² is a critical operational ceiling. Exceeding this current density can rapidly degrade the platinum layer through physical wear and dissolution, leading to a swift decline in performance and electrode failure. Operating well below this limit is key to maximizing service life.
Understanding the Electrochemical Performance
The material specifications translate directly into distinct performance characteristics that make this electrode suitable for a wide range of applications.
High Catalytic Activity
The platinum surface is highly active, efficiently facilitating reactions. This makes it an ideal choice for processes that require high efficiency and low energy consumption.
Versatility as Anode and Cathode
This electrode possesses a high oxygen evolution potential and a low hydrogen evolution potential. This unique combination means it performs efficiently as an anode (in processes like oxygen generation) and as a cathode (in processes like hydrogen generation), making it exceptionally versatile for applications like water electrolysis.
Suitability for Polarity Reversal
The ability to withstand polarity reversal is a sign of a robust design. This feature is useful in applications that use periodic polarity switching to clean electrode surfaces and maintain efficiency over time.
Understanding the Trade-offs
Selecting the right electrode requires balancing competing factors. There is no single "best" configuration; the optimal choice depends entirely on your application's demands.
Cost vs. Longevity
This is the primary trade-off. A thicker platinum coating (e.g., 5-10μm) is significantly more expensive but will provide a much longer service life, especially under aggressive operating conditions. A thin coating (0.3-1.0μm) is a cost-effective option for less demanding applications or for initial prototyping, but it will require more frequent replacement.
Substrate Shape vs. Efficiency
A mesh substrate offers a much higher surface area-to-volume ratio compared to a solid plate of the same external dimensions. This increases the active area for reactions, improving overall process efficiency. However, a simple plate may be easier to clean and more mechanically rigid.
Aggressive Operation vs. Electrode Life
Pushing the electrode toward its maximum current density limit of 20,000 A/m² will maximize immediate output. However, this comes at the steep cost of accelerated wear on the platinum layer. Operating at a more conservative current density will dramatically extend the electrode's functional lifespan.
Selecting the Right Electrode for Your Application
Use your primary objective to guide your selection of the electrode's final specifications.
- If your primary focus is maximum service life in a harsh environment: Opt for a thicker platinum coating (closer to 10μm) and ensure your operational current density is well below the 20,000 A/m² limit.
- If your primary focus is cost-sensitive prototyping or a low-intensity process: A thinner coating (0.3-2.0μm) may be sufficient, but you must carefully monitor performance for signs of degradation.
- If your primary focus is maximizing process efficiency and throughput: Choose a mesh substrate to increase the active surface area and carefully balance current density against your acceptable electrode replacement cycle.
By understanding how each specification impacts performance, you can select an electrode that provides the optimal balance of efficiency, longevity, and cost for your specific goal.
Summary Table:
| Specification | Details |
|---|---|
| Substrate | High-purity titanium (plate, mesh, tube, rod) |
| Platinum Coating Thickness | 0.3 to 10 micrometers (μm) |
| Precious Metal Content | > 15 grams per square meter (g/m²) |
| Maximum Current Density | < 20,000 amperes per square meter (A/m²) |
| Key Features | High catalytic activity, versatile (anode/cathode), suitable for polarity reversal |
Ready to optimize your electrochemical process with the right Platinum-Titanium Electrode?
KINTEK specializes in high-performance lab equipment and consumables, providing durable and efficient electrodes tailored to your specific application needs—whether for water electrolysis, research, or industrial processes. Our expertise ensures you get the ideal balance of cost, longevity, and efficiency.
Contact our experts today for a personalized consultation and quote!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
People Also Ask
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results



















