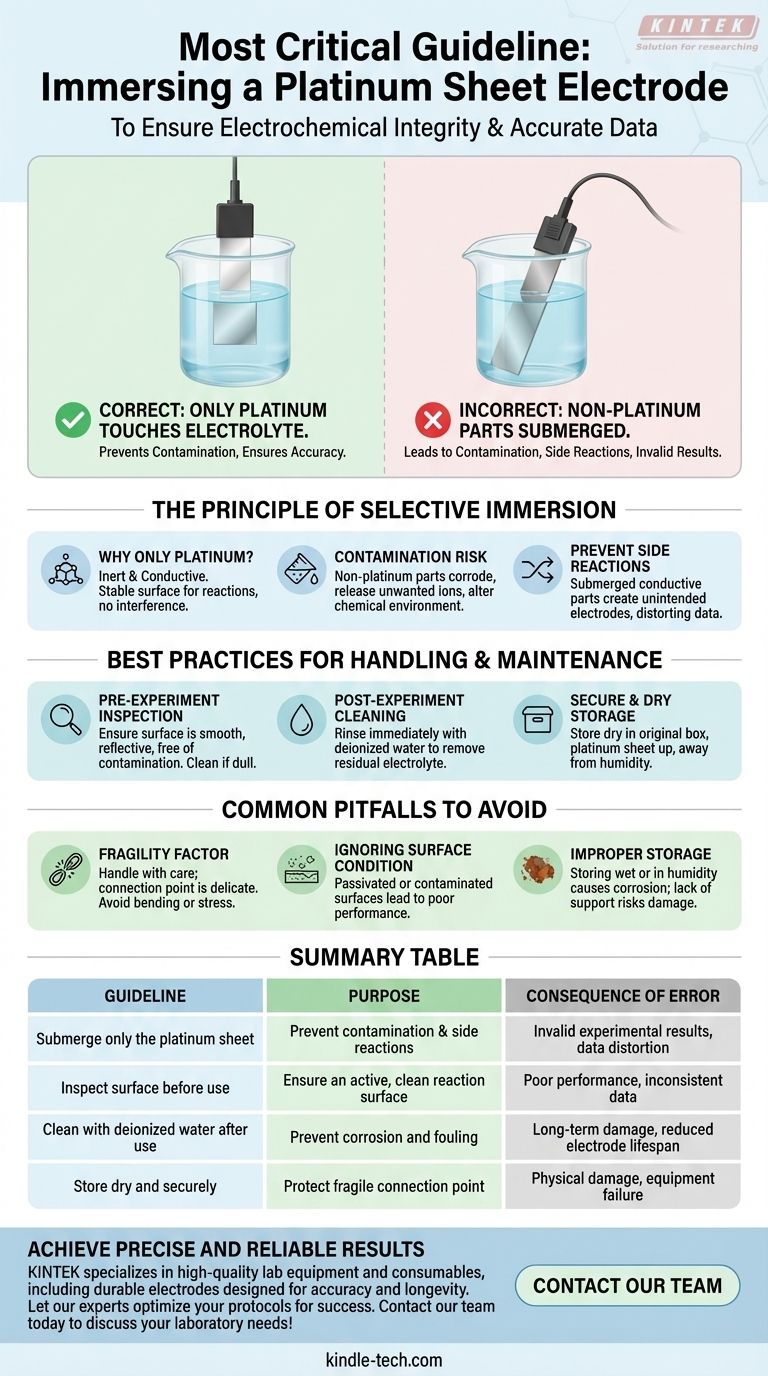
When immersing a platinum sheet electrode, the single most critical guideline is to ensure that only the platinum portion makes contact with the electrolyte. All other parts, particularly the connection point and any protective casing, are strictly prohibited from touching the solution. Submerging these components can lead to immediate contamination and invalid experimental results.
Proper electrode immersion is not simply a handling instruction; it is a fundamental requirement for electrochemical integrity. Submerging non-platinum parts introduces contaminants and unwanted side reactions that compromise the accuracy of your measurements.

The Principle of Selective Immersion
The rule to only submerge the platinum sheet is rooted in the core principles of electrochemistry. Ignoring it fundamentally invalidates the experiment.
Why Only Platinum Touches the Solution
Platinum is chosen for its inertness and conductivity. It provides a stable surface for electrochemical reactions to occur without itself reacting with the electrolyte.
The other parts of the electrode, such as the wire lead, solder joints, and plastic or glass casing, are not designed for this purpose and will interfere with the measurement.
The Risk of Contamination
Materials used in the electrode's construction, other than the platinum itself, can corrode or dissolve when exposed to an electrolyte.
This process releases unwanted ions into your solution, contaminating the experiment and altering the very chemical environment you are trying to study.
Preventing Unwanted Side Reactions
If conductive parts other than the platinum sheet are submerged, they can act as secondary, unintended electrodes.
This creates parallel electrical pathways and promotes side reactions, which draw current away from the intended process and distort your data. The platinum sheet must be the sole interface for the intended reaction.
Best Practices for Handling and Maintenance
Proper immersion is part of a larger protocol for ensuring the electrode's performance and longevity.
Pre-Experiment Inspection
Before each use, visually inspect the platinum surface. It should be smooth, reflective, and free of any visible contamination or discoloration. If the surface appears dull or fouled, it must be cleaned and re-treated.
Post-Experiment Cleaning
Immediately after an experiment concludes, rinse the electrode thoroughly with deionized water. This removes any residual electrolyte, preventing it from drying on the surface and causing corrosion or fouling.
Secure and Dry Storage
After cleaning and drying, store the electrode in a clean, dry environment. To prevent physical damage, especially to the fragile connection point, place it in its original storage box with the platinum sheet facing upwards.
Common Pitfalls to Avoid
Even experienced users can make mistakes that compromise results or damage the equipment. Being aware of these pitfalls is crucial for consistent performance.
The Fragility Factor
Platinum sheet electrodes are delicate. The connection between the sheet and the conducting wire is a common point of failure. Always handle the electrode with care, avoiding any bending of the sheet or stress on the connection point.
Ignoring Surface Condition
Correct immersion is pointless if the electrode surface is not active. A passivated or contaminated surface will not function correctly, leading to poor performance and inconsistent data. Regular cleaning is not optional.
Improper Storage Leads to Damage
Storing the electrode while it is still wet or in a humid environment can lead to long-term corrosion of the non-platinum components. Likewise, storing it without proper support can easily damage the fragile connection.
Making the Right Choice for Your Goal
Your handling protocol should directly support your experimental objectives.
- If your primary focus is experimental accuracy: Your first and most critical check is to verify that only the platinum sheet is submerged to prevent contamination and side reactions.
- If your primary focus is equipment longevity: Implement a strict post-experiment cleaning and dry storage protocol to prevent corrosion and physical damage to the electrode's non-platinum parts.
- If you are troubleshooting inconsistent data: Before re-calibrating instruments, confirm the electrode's immersion depth and surface cleanliness, as these are the most common sources of error.
Proper handling is not just about protecting the equipment; it is the foundation of reliable electrochemical measurement.
Summary Table:
| Guideline | Purpose | Consequence of Error |
|---|---|---|
| Submerge only the platinum sheet | Prevent contamination and side reactions | Invalid experimental results, data distortion |
| Inspect surface before use | Ensure an active, clean reaction surface | Poor performance, inconsistent data |
| Clean with deionized water after use | Prevent corrosion and fouling | Long-term damage, reduced electrode lifespan |
| Store dry and securely | Protect fragile connection point | Physical damage, equipment failure |
Achieve precise and reliable results in your electrochemical experiments. Proper electrode handling is fundamental to data integrity. KINTEK specializes in high-quality lab equipment and consumables, including durable electrodes designed for accuracy and longevity. Let our experts help you select the right tools and optimize your protocols for success. Contact our team today to discuss your laboratory needs!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Sheet Electrode for Battery Lab Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Electrochemical Sheet Electrode Gold Electrode
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment



















