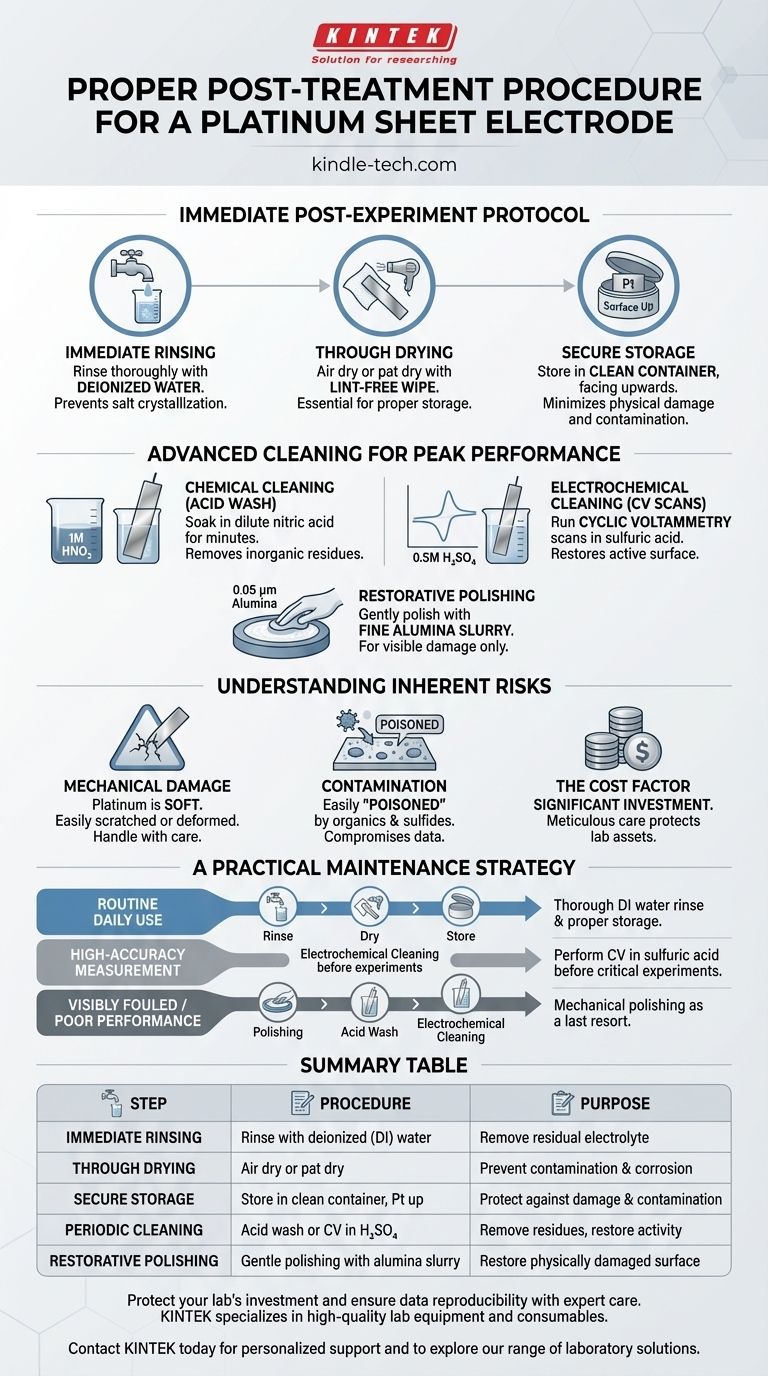
The proper post-treatment procedure for a platinum sheet electrode is a two-part process of immediate cleaning and secure storage. Immediately after an experiment, the electrode must be thoroughly rinsed with deionized water to remove any residual electrolyte, then allowed to dry completely. It should then be stored in a clean, dry container, ideally its original box, with the delicate platinum surface facing upwards to prevent physical damage or contamination.
Your platinum electrode's surface is its most critical asset. Post-treatment is not just about cleaning; it's the first step in a continuous maintenance cycle designed to preserve this pristine surface, ensuring the accuracy and reproducibility of your future experiments.

The Immediate Post-Experiment Protocol
The steps you take in the minutes after your experiment concludes are crucial for preventing long-term damage and contamination.
Step 1: Immediate Rinsing
As soon as the experiment is finished, rinse the electrode surface thoroughly with deionized (DI) water.
This step is non-negotiable. It prevents residual electrolyte salts from crystallizing on the platinum surface, which can be difficult to remove later and can interfere with subsequent measurements.
Step 2: Thorough Drying
After rinsing, allow the electrode to air dry completely or gently pat it dry with a lint-free laboratory wipe.
Moisture can attract airborne dust and contaminants or lead to slow corrosion of the electrode's connection points. A perfectly dry electrode is essential for proper storage.
Step 3: Secure Storage
Place the dry electrode in a dedicated, clean container. The original packaging is often ideal as it is designed to protect the electrode body and its fragile connection point.
Always store the electrode with the platinum sheet facing upwards. This minimizes the risk of scratches, dents, or contact with contaminating surfaces.
Advanced Cleaning for Peak Performance
While immediate rinsing is sufficient for daily use, periodic, more intensive cleaning is required to maintain the electrode's electrochemical activity and ensure reproducible results.
Chemical Cleaning (Acid Washing)
For removing inorganic residues or light metal deposition, an acid wash is effective.
Soak the platinum sheet in a dilute acid, such as 1M nitric acid (HNO₃), for a few minutes. Follow this with an exhaustive rinse using deionized water.
Electrochemical Cleaning
This is the gold standard for creating a reproducibly clean and active platinum surface. It is often performed before a critical experiment.
The process involves running cyclic voltammetry (CV) scans in a dilute acid electrolyte, typically 0.5M sulfuric acid (H₂SO₄). You scan the potential repeatedly until the characteristic hydrogen and oxygen adsorption/desorption peaks are stable and well-defined, indicating a clean surface.
Mechanical Polishing
This is a restorative, not routine, procedure used only when the electrode surface is visibly scratched or has stubborn, adhered contamination.
Gently polish the surface on a polishing pad with a fine alumina powder slurry (e.g., 0.05 µm). Afterwards, the electrode must be sonicated in DI water to remove all polishing particles and then electrochemically cleaned to restore its activity.
Understanding the Inherent Risks
Proper care is critical because platinum electrodes are both expensive and sensitive. Understanding their vulnerabilities is key to extending their lifespan.
Vulnerability to Mechanical Damage
Platinum is a very soft metal. The thin foil of an electrode can be easily scratched, bent, or deformed. Always handle the electrode with care and never touch the surface with bare hands or hard tools.
High Susceptibility to Contamination
The catalytically active surface of platinum is easily "poisoned" by a wide range of substances, especially organics and sulfides. This contamination blocks active sites and will severely compromise your electrochemical data.
The Cost Factor
Platinum electrodes represent a significant financial investment. Meticulous care is not just good scientific practice; it is a direct measure to protect your lab's assets and avoid unnecessary replacement costs.
A Practical Maintenance Strategy
Your cleaning protocol should match your experimental needs. Use this guide to determine the appropriate level of care.
- If your primary focus is routine daily use: A thorough rinse with DI water, followed by proper drying and storage, is sufficient after each experiment.
- If your primary focus is high-accuracy measurement: Perform electrochemical cleaning in sulfuric acid before each critical experiment to guarantee a standardized, active surface.
- If your electrode is visibly fouled or performing poorly: Use mechanical polishing as a last resort to restore the physical surface, then follow up with acid washing and electrochemical cleaning to fully reactivate it.
Ultimately, treating your electrode with consistent care is the single best way to ensure it produces reliable data for years to come.
Summary Table:
| Step | Procedure | Purpose |
|---|---|---|
| Immediate Rinsing | Rinse with deionized (DI) water. | Remove residual electrolyte to prevent salt crystallization. |
| Thorough Drying | Air dry or pat dry with a lint-free wipe. | Prevent contamination and corrosion from moisture. |
| Secure Storage | Store in a clean, dry container with the Pt surface facing up. | Protect against physical damage and contamination. |
| Periodic Cleaning | Acid wash (1M HNO₃) or electrochemical cleaning (CV in 0.5M H₂SO₄). | Remove inorganic/organic residues and restore electrochemical activity. |
| Restorative Polishing | Gentle polishing with fine alumina slurry (for visible damage only). | Restore a physically damaged surface. |
Protect your lab's investment and ensure data reproducibility with expert care. Platinum electrodes are a critical asset for accurate electrochemical measurements. KINTEK specializes in high-quality lab equipment and consumables, serving the precise needs of research and quality control laboratories. Let our experts help you maintain your instrumentation for peak performance.
Contact KINTEK today for personalized support and to explore our range of laboratory solutions.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Electrochemical Sheet Electrode Gold Electrode
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs



















