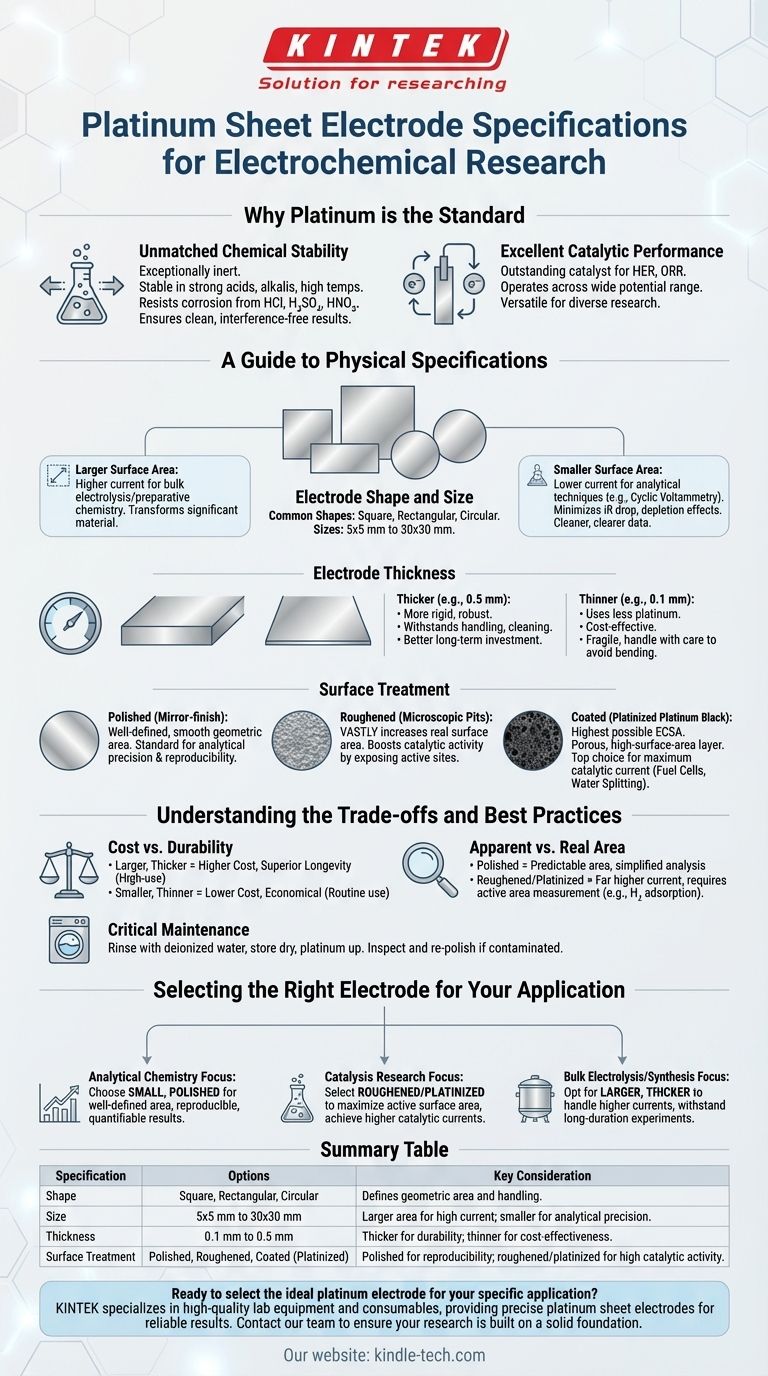
At a glance, platinum sheet electrodes are available in square, rectangular, or circular shapes, with sizes ranging from 5x5 mm to 30x30 mm and a standard thickness between 0.1 mm and 0.5 mm. Critically, their surfaces can be polished for analytical precision, roughened to increase surface area, or coated for enhanced catalytic activity.
Selecting the correct platinum electrode is not about finding the "best" one, but about matching its physical specifications—size, thickness, and surface texture—to the specific demands of your electrochemical experiment. This choice directly dictates the electrode's performance, durability, and the quality of your results.

Why Platinum is the Standard
Before examining specifications, it's essential to understand why platinum is a benchmark material in electrochemistry. Its value comes from a unique combination of properties.
Unmatched Chemical Stability
Platinum is exceptionally inert. It remains stable in the presence of strong acids, strong alkalis, and at high temperatures, showing remarkable resistance to corrosion from media like hydrochloric, sulfuric, and nitric acid.
This stability ensures that the electrode itself does not interfere with the electrochemical reaction you are studying, providing a clean and reliable experimental environment.
Excellent Catalytic Performance
Platinum is an outstanding catalyst for many key electrochemical reactions, including the hydrogen evolution reaction (HER) and the oxygen reduction reaction (ORR).
It can operate effectively across a wide potential range, making it a versatile and ideal choice for a vast array of electrochemical research and applications.
A Guide to Physical Specifications
Each specification of a platinum sheet electrode serves a distinct purpose. Understanding these details allows you to select an electrode that is optimized for your goal.
Electrode Shape and Size
The dimensions of the electrode directly relate to its total surface area. Common shapes include square, rectangular, and circular sheets, with sizes typically from 5x5 mm to 30x30 mm.
A larger surface area allows for a higher total current at a given potential. This is ideal for bulk electrolysis or preparative chemistry, where the goal is to transform a significant amount of material.
A smaller surface area is preferred for analytical techniques like cyclic voltammetry. It keeps currents low, minimizing solution resistance (iR drop) and depletion effects, which leads to cleaner, more easily interpreted data.
Electrode Thickness
Thickness, typically ranging from 0.1 mm to 0.5 mm, is primarily a balance between durability and cost.
A thicker electrode (e.g., 0.5 mm) is more rigid and mechanically robust. It can withstand more handling and cleaning cycles, making it a better long-term investment for high-use environments.
A thinner electrode (e.g., 0.1 mm) uses less platinum, making it more cost-effective. However, it is more fragile and must be handled with greater care to avoid bending or damage.
Surface Treatment
The texture of the platinum surface is one of its most critical specifications, as it determines the electrochemically active surface area (ECSA).
- Polished: A mirror-finish surface provides a well-defined, smooth geometric area. This is the standard for analytical studies where reproducibility and accurate calculation of current density are paramount.
- Roughened: Mechanically or chemically roughening the surface creates microscopic pits and peaks. This vastly increases the real surface area compared to the geometric area, boosting catalytic activity by exposing more active sites.
- Coated (Platinized): This involves electrodepositing a layer of platinum black, a porous, high-surface-area form of platinum. This method achieves the highest possible ECSA, making it the top choice for applications demanding maximum catalytic current, such as fuel cell research or high-efficiency water splitting.
Understanding the Trade-offs and Best Practices
Choosing an electrode involves practical considerations, and maintaining it properly is as important as the initial selection.
Cost vs. Durability
A larger, thicker platinum electrode represents a significant initial investment but offers superior longevity. For routine, less demanding work, a thinner, smaller electrode may be a more economical choice.
Apparent Area vs. Real Surface Area
A polished electrode's predictable surface area simplifies data analysis. In contrast, a roughened or platinized electrode delivers far higher current for a given size but requires techniques like hydrogen adsorption/desorption to measure its true active area.
The Critical Role of Maintenance
Regardless of its specifications, an electrode's performance will degrade without proper care.
Always rinse the electrode with deionized water immediately after use to remove electrolyte residue. Store it dry, preferably in its original case, with the platinum sheet facing up to protect the fragile connection point. Before use, visually inspect the surface for contamination and re-polish or clean it if necessary.
Selecting the Right Electrode for Your Application
Use your experimental goal as the primary guide for your selection.
- If your primary focus is analytical chemistry: Choose a smaller, polished electrode for its well-defined surface area, which ensures reproducible and easily quantifiable results.
- If your primary focus is catalysis research: Select a roughened or platinized electrode to maximize the active surface area and achieve higher catalytic currents for studying reaction kinetics.
- If your primary focus is bulk electrolysis or synthesis: Opt for a larger, thicker electrode that can handle higher currents and withstand the rigors of long-duration experiments.
Ultimately, choosing the right electrode specification is the foundational step toward achieving reliable and reproducible electrochemical data.
Summary Table:
| Specification | Options | Key Consideration |
|---|---|---|
| Shape | Square, Rectangular, Circular | Defines geometric area and handling. |
| Size | 5x5 mm to 30x30 mm | Larger area for high current; smaller for analytical precision. |
| Thickness | 0.1 mm to 0.5 mm | Thicker for durability; thinner for cost-effectiveness. |
| Surface Treatment | Polished, Roughened, Coated (Platinized) | Polished for reproducibility; roughened/platinized for high catalytic activity. |
Ready to select the ideal platinum electrode for your specific application?
KINTEK specializes in high-quality lab equipment and consumables, providing researchers with the precise platinum sheet electrodes they need for reliable electrochemical results. Whether your work requires analytical precision, maximum catalytic activity, or robust synthesis capabilities, our experts can help you match the perfect specifications to your experiment.
Contact our team today to discuss your requirements and ensure your research is built on a solid foundation.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
People Also Ask
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What is a common use for a platinum sheet electrode? As a Reliable Counter Electrode in Electrochemical Cells
- How should a platinum wire/rod electrode be installed? Ensure Accurate Electrochemical Measurements
- How can a worn or scratched platinum disk electrode surface be restored? Achieve a Mirror Finish for Reliable Data



















