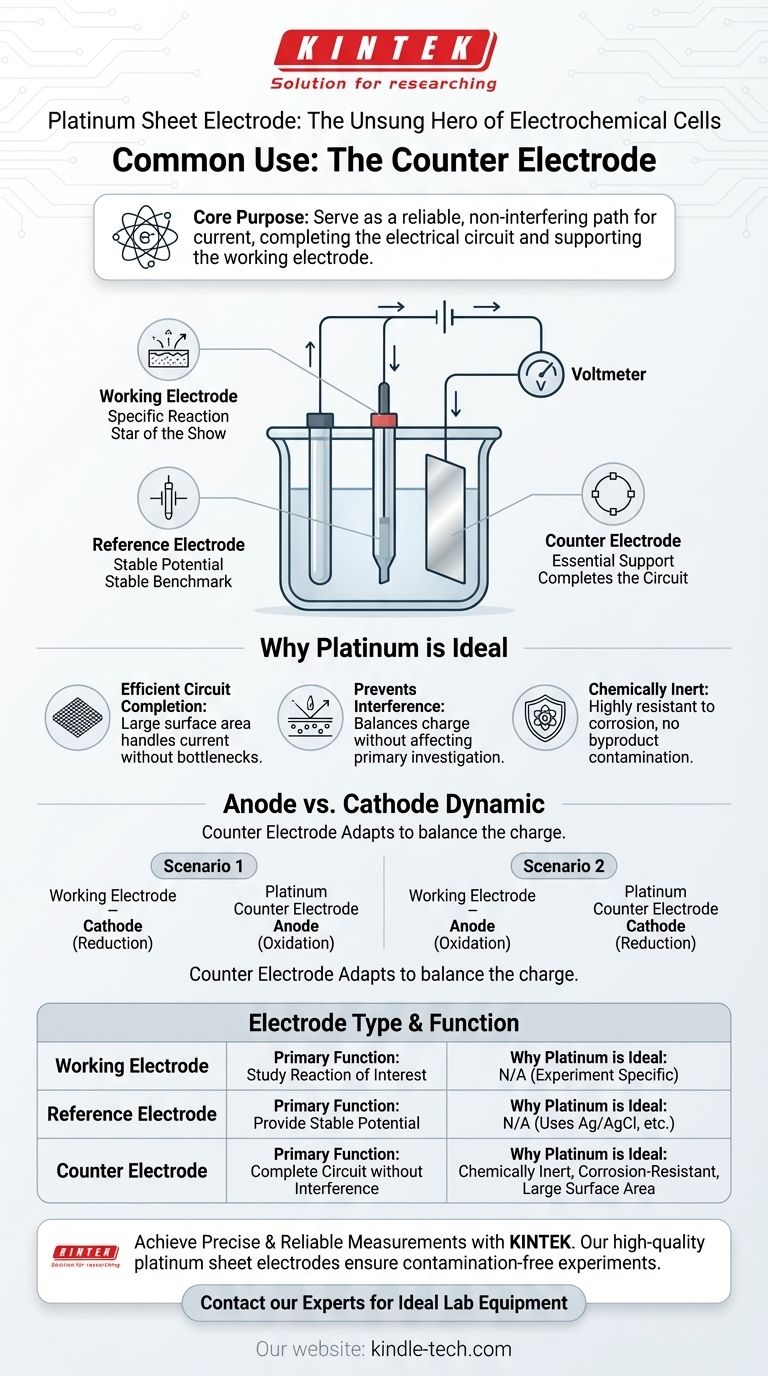
In an electrochemical cell, a platinum sheet electrode is most commonly used as the counter electrode, also known as the auxiliary electrode. Its primary function is to complete the electrical circuit, allowing the reaction of interest at the working electrode to be measured accurately and without interference.
The core purpose of a platinum counter electrode is not to be studied, but to serve as a reliable, non-interfering path for current. It supports the main electrochemical event occurring at the working electrode by balancing the flow of electrons in the cell.

Deconstructing the Three-Electrode System
To understand the platinum sheet's role, you must first understand the standard three-electrode setup used in modern electrochemistry. Each electrode has a distinct and critical job.
The Working Electrode: The Star of the Show
The working electrode is the surface where the specific electrochemical reaction you are studying takes place. It is the center of the experiment, analogous to a weight-loss coupon in a corrosion test.
The Reference Electrode: The Stable Benchmark
The reference electrode provides a stable, known potential that does not change during the experiment. All potential measurements of the working electrode are made relative to this constant benchmark, ensuring accuracy.
The Counter Electrode: The Essential Support
The counter electrode acts as a source or a sink for electrons to complete the circuit. It passes all the current needed by the working electrode so that the sensitive reference electrode doesn't have to, which would destabilize its potential.
Why Platinum is an Ideal Counter Electrode
A platinum sheet is chosen for this supporting role due to a unique combination of properties that prevent it from disrupting the experiment.
Completing the Circuit Efficiently
The primary job of the counter electrode is to ensure current can flow to the working electrode. The large surface area of a platinum sheet allows it to handle the necessary current without becoming a bottleneck in the system.
Preventing Interference
The chemical reactions that happen on the counter electrode are simply the inverse of those at the working electrode. They are necessary to balance the charge but are not part of the primary investigation.
The Importance of Inertness
Platinum is a noble metal, meaning it is chemically inert and highly resistant to corrosion. This is its most critical feature as a counter electrode. It will not dissolve or react in a way that creates byproducts that could contaminate the solution and interfere with the sensitive measurements at the working electrode.
Understanding the Anode vs. Cathode Dynamic
It's important to clarify that an electrode's role as anode or cathode is not fixed. This is determined by the specific experiment being run.
A Role Determined by the Experiment
The anode is where oxidation occurs (electrons leave), and the cathode is where reduction occurs (electrons enter).
The Counter Electrode Adapts
If the working electrode is functioning as a cathode (reduction), the counter electrode will act as the anode (oxidation) to balance the charge, and vice-versa. The platinum counter electrode simply takes on whatever opposing role is necessary to keep the system running.
Making the Right Choice for Your Goal
In any electrochemical setup, your objective determines your choice of electrode.
- If your primary focus is studying a specific reaction: You need a working electrode made of a material directly relevant to your experiment.
- If your primary focus is establishing a stable potential for measurement: You need a dedicated reference electrode to serve as a reliable zero point.
- If your primary focus is completing the circuit without introducing variables: You need an inert counter electrode, for which a platinum sheet is the industry-standard choice.
Ultimately, the platinum counter electrode is the silent partner that enables precise and repeatable electrochemical analysis.
Summary Table:
| Electrode Type | Primary Function | Why Platinum is Ideal |
|---|---|---|
| Working Electrode | The surface where the reaction of interest is studied. | N/A (Material is experiment-specific) |
| Reference Electrode | Provides a stable, known potential for accurate measurement. | N/A (Uses specialized materials like Ag/AgCl) |
| Counter Electrode | Completes the electrical circuit without interfering. | Chemically inert, corrosion-resistant, and provides a large surface area. |
Achieve precise and reliable electrochemical measurements with KINTEK.
Our high-quality platinum sheet electrodes are designed to be the perfect, inert counter electrode for your lab's three-electrode cells, ensuring your experiments are free from contamination and interference.
Ready to enhance your electrochemical analysis? Contact our experts today to find the ideal lab equipment and consumables for your specific research needs.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
People Also Ask
- What are the standard specifications for platinum wire and rod electrodes? Select the Right Form Factor for Your Experiment
- What are platinum electrodes used for? Essential Uses in Science, Medicine, and Industry
- What can cause poisoning of a platinum disk electrode and how can it be prevented? Ensure Reliable Electrochemical Data
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements



















