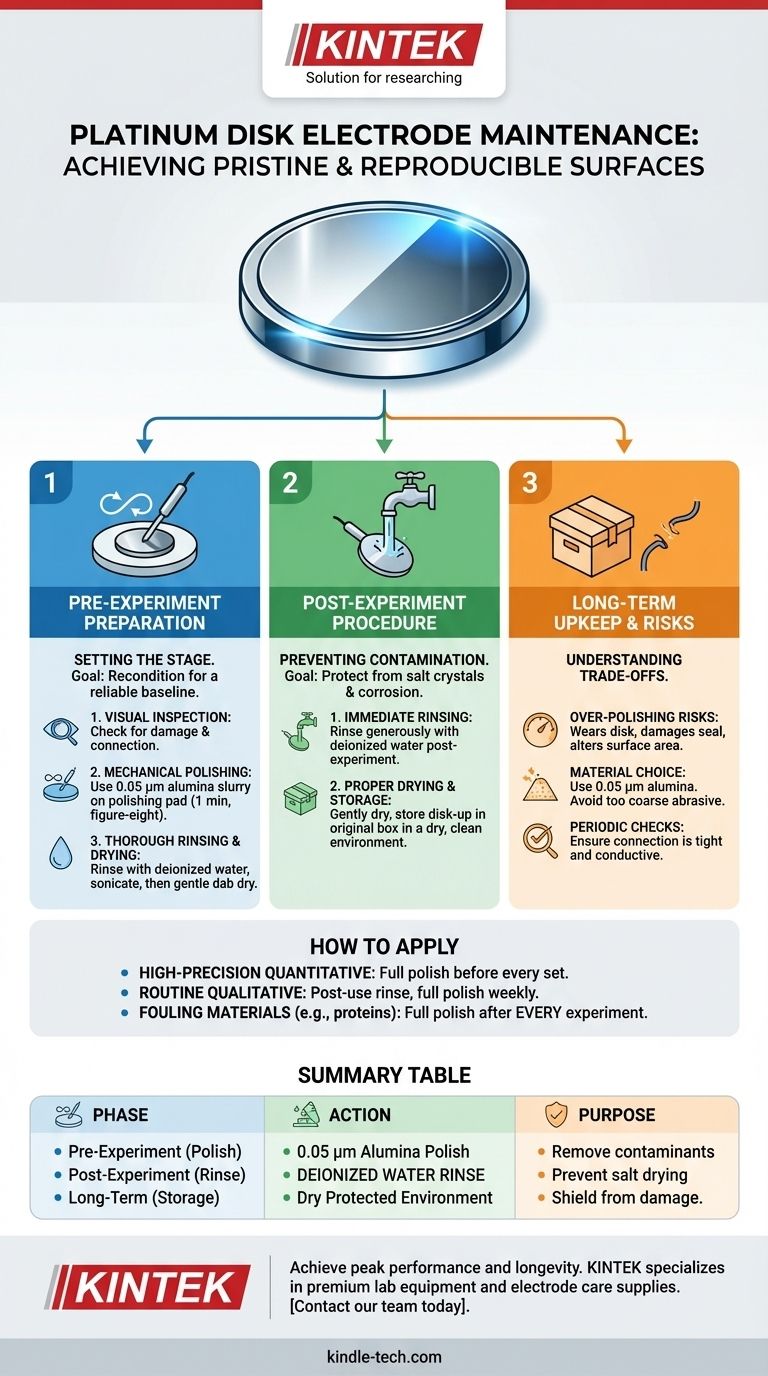
To ensure accurate and reproducible results, regular maintenance for a platinum disk electrode centers on a disciplined routine of cleaning, polishing, and proper storage. Immediately after any experiment, the electrode must be rinsed with deionized water to remove residual electrolyte. For a deeper clean or to restore performance, the surface is mechanically polished, typically with a 0.05 µm alumina slurry, before being thoroughly rinsed and stored in a dry, protected environment.
A platinum electrode's performance is dictated entirely by the state of its surface. The core purpose of all maintenance is to remove contaminants and ensure you are working with a consistently clean, active, and electrochemically reproducible platinum surface for every experiment.

The Goal: A Pristine and Reproducible Surface
The data you collect is a direct reflection of the electrochemical reactions occurring at your electrode's surface. A contaminated or oxidized surface will produce noisy, shifted, or entirely incorrect results, invalidating your work.
Why Surface Condition is Critical
Any substance other than your target analyte that interacts with the platinum can interfere with your measurement. This includes adsorbed ions from a previous experiment, surface oxides that form on the platinum, or residue from polishing materials.
The Three Phases of Maintenance
Effective electrode care can be broken down into three distinct phases: the preparation you perform before an experiment, the immediate cleanup you do after an experiment, and the periodic checks you conduct as long-term upkeep.
Pre-Experiment Preparation: Setting the Stage
Before every critical experiment, you must recondition the electrode surface to establish a reliable baseline. This is not just cleaning; it is a full reset.
Step 1: Visual Inspection
Begin with a simple visual check. Look for any obvious physical damage, deep scratches, or delamination of the platinum disk from its insulating shroud. Ensure the electrical connection point is secure and free of corrosion.
Step 2: Mechanical Polishing
Polishing is the most critical step for restoring a poorly performing electrode. It mechanically removes a microscopic layer from the surface, along with any embedded impurities or passive oxide layers.
Place a small amount of 0.05 µm alumina powder slurry on a polishing pad. Gently press the electrode face down onto the pad and move it in a figure-eight pattern for about one minute. This ensures even wear and a mirror-like finish.
Step 3: Thorough Rinsing and Drying
After polishing, the electrode is covered in alumina slurry that must be completely removed. Rinse it thoroughly with deionized water. You may also sonicate the electrode in deionized water for a few minutes to dislodge any stubborn particles from microscopic crevices.
After rinsing, gently dry the electrode surface by dabbing it with a clean, lint-free cloth or filter paper. Do not rub the surface, as this can reintroduce contaminants.
Post-Experiment Procedure: Preventing Contamination
What you do in the five minutes after an experiment concludes has the greatest impact on the electrode's long-term health and readiness for the next use.
Immediate Rinsing is Non-Negotiable
As soon as the electrode is removed from the electrochemical cell, rinse it generously with deionized water. This prevents the electrolyte from drying on the surface, which can lead to the formation of stubborn salt crystals and corrosion.
Proper Drying and Storage
Once rinsed and gently dried with filter paper, the electrode must be stored correctly. The best practice is to place it back in its original storage box, with the platinum disk facing upwards.
Store the box in a dry, clean environment away from corrosive fumes, high humidity, or extreme temperatures. This protects the delicate surface and the sensitive electrical connection point from damage and degradation.
Understanding the Trade-offs and Risks
While maintenance is essential, improper techniques can do more harm than good. Understanding the risks is key to preserving your electrode.
The Danger of Over-Polishing
Polishing is an abrasive process. Over-polishing or using excessive force can wear down the platinum disk over time, altering its precise surface area. More critically, it can damage the seal between the platinum and the insulating shroud, leading to unwanted crevice corrosion and unreliable data.
Choosing the Right Polishing Material
While 0.05 µm alumina is a common standard, even finer diamond pastes can be used for sensitive applications. Conversely, using a polishing abrasive that is too coarse (e.g., 1.0 µm) can leave deep scratches that are difficult to clean and alter the electrode's electrochemical behavior.
Periodic Connection Checks
Vibrations or frequent handling can cause the wire connection to loosen over time, leading to high resistance or an open circuit. Periodically check that the connection is tight and conductive to prevent sudden experiment failure.
How to Apply This to Your Project
Your maintenance frequency should match the demands of your experimental work. A one-size-fits-all approach is not effective.
- If your primary focus is high-precision quantitative analysis: Perform a full mechanical polish and rinse cycle before every set of crucial measurements.
- If your primary focus is routine qualitative screening: A thorough rinse with deionized water after each use is sufficient, with a full polish performed weekly or whenever performance degrades.
- If you are working with materials known to foul the electrode (e.g., polymers, proteins): A full polish and aggressive cleaning protocol is mandatory after every single experiment.
Ultimately, disciplined electrode maintenance is the foundation of trustworthy electrochemical data.
Summary Table:
| Maintenance Phase | Key Action | Purpose |
|---|---|---|
| Pre-Experiment | Mechanical polishing with 0.05 µm alumina slurry | Remove contaminants and oxides; create a fresh, active surface. |
| Post-Experiment | Immediate rinsing with deionized water | Prevent electrolyte from drying and forming corrosive salts. |
| Long-Term Upkeep | Proper storage in a dry, protected environment | Shield the sensitive surface from damage and contamination. |
Achieve peak performance and longevity for your laboratory electrodes. Proper maintenance is critical, but so is starting with high-quality equipment. KINTEK specializes in premium lab equipment and consumables, including the reliable supplies you need for consistent electrode care. Let our experts help you select the right tools for your specific electrochemical applications. Contact our team today to discuss your laboratory needs and ensure your data is always accurate.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- What are the functions of platinum sheet and Ag/AgCl electrodes in corrosion testing? Master Electrochemical Precision
- What is a common use for a platinum sheet electrode? As a Reliable Counter Electrode in Electrochemical Cells
- How should a platinum wire/rod electrode be installed? Ensure Accurate Electrochemical Measurements
- What are platinum electrodes used for? Essential Uses in Science, Medicine, and Industry



















